 To be evaluated to determine if monoclonal antibody treatment might be appropriate, please contact your primary care provider. Its worth noting that because Paxlovid is still being monitored in the real world, it is possible that all of the risks are not yet known. COVID-19 vaccines available in the United States effectively protect people from getting seriously ill, being hospitalized, and even dyingespecially people who are boosted. It helps that the pills are packaged in a dose card, basically a medication blister pack that allows you to punch out the pills as needed. WebThe 2 therapies offered at the COVID Monoclonal Antibody Infusion Clinic are available to people who have tested positive for COVID-19 but have not yet developed severe Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. People with certain conditions, such as: cancer; kidney, liver, lung or sickle cell disease; dementia; diabetes; down syndrome; heart conditions; HIV infection; certain mental health conditions; current or former smoker; organ transplant recipient; stroke; substance use disorder; tuberculosis. Home Heres everything you need to know about what the treatment can and cannot do, and the critical difference between getting a treatment and getting a vaccine. Its always been the Achilles heel of these antiviral drugs that most people dont get testedor they dont have access to testing.. Antiviral, Start as soon as possible; must begin within 5 days of when symptoms start, Start as soon as possible; must begin within 7 days of when symptoms start, Intravenous (IV) infusions at a healthcare facility for 3 consecutive days, Who (The FDA has provided a fact sheet on Paxlovid with a full list of known side effects.). Dont delay: Treatment must be started within days of when you first develop symptoms to be effective. As of January 26, 2023, EVUSHELDTM is not currently authorized for emergency use because it is unlikely to be active against the majority of SARS-CoV-2 variants circulating in the United States. The more underlying medical conditions a person has, the higher their risk for developing a severe case of COVID-19, according to the CDC. Can I drink alcohol while taking antibiotics? Pfizer recommends reporting it to them on its portal for adverse events associated with Paxlovid. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. Serious and unexpected side effects may occur. In November 2022, the CDC reported on a real-world study that showed adults who took Paxlovid within five days of a COVID-19 diagnosis had a 51% lower hospitalization rate within the next 30 days than those who were not given the drug. A UCHealth provider will determine if you qualify for treatment. There is a long list of medications Paxlovid may interact with, and in some cases, doctors may not prescribe Paxlovid because these interactions may cause serious complications. The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID. A UCHealth provider will determine if you qualify for treatment. To get the treatment administered, youll get antibodies either by four subcutaneous injections in areas like your arms and belly in quick succession, or the treatment will be given to you through a vein intravenously that can take between 20 minutes to an hour or longer. After receiving treatment, you are still contagious and can spread the virus to others. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. Both are prescription-only oral antiviral pills given early in illness. The United States FDA has made this treatment available under an emergency access mechanism called an Emergency Use Authorization (EUA).
To be evaluated to determine if monoclonal antibody treatment might be appropriate, please contact your primary care provider. Its worth noting that because Paxlovid is still being monitored in the real world, it is possible that all of the risks are not yet known. COVID-19 vaccines available in the United States effectively protect people from getting seriously ill, being hospitalized, and even dyingespecially people who are boosted. It helps that the pills are packaged in a dose card, basically a medication blister pack that allows you to punch out the pills as needed. WebThe 2 therapies offered at the COVID Monoclonal Antibody Infusion Clinic are available to people who have tested positive for COVID-19 but have not yet developed severe Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. People with certain conditions, such as: cancer; kidney, liver, lung or sickle cell disease; dementia; diabetes; down syndrome; heart conditions; HIV infection; certain mental health conditions; current or former smoker; organ transplant recipient; stroke; substance use disorder; tuberculosis. Home Heres everything you need to know about what the treatment can and cannot do, and the critical difference between getting a treatment and getting a vaccine. Its always been the Achilles heel of these antiviral drugs that most people dont get testedor they dont have access to testing.. Antiviral, Start as soon as possible; must begin within 5 days of when symptoms start, Start as soon as possible; must begin within 7 days of when symptoms start, Intravenous (IV) infusions at a healthcare facility for 3 consecutive days, Who (The FDA has provided a fact sheet on Paxlovid with a full list of known side effects.). Dont delay: Treatment must be started within days of when you first develop symptoms to be effective. As of January 26, 2023, EVUSHELDTM is not currently authorized for emergency use because it is unlikely to be active against the majority of SARS-CoV-2 variants circulating in the United States. The more underlying medical conditions a person has, the higher their risk for developing a severe case of COVID-19, according to the CDC. Can I drink alcohol while taking antibiotics? Pfizer recommends reporting it to them on its portal for adverse events associated with Paxlovid. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. Serious and unexpected side effects may occur. In November 2022, the CDC reported on a real-world study that showed adults who took Paxlovid within five days of a COVID-19 diagnosis had a 51% lower hospitalization rate within the next 30 days than those who were not given the drug. A UCHealth provider will determine if you qualify for treatment. There is a long list of medications Paxlovid may interact with, and in some cases, doctors may not prescribe Paxlovid because these interactions may cause serious complications. The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID. A UCHealth provider will determine if you qualify for treatment. To get the treatment administered, youll get antibodies either by four subcutaneous injections in areas like your arms and belly in quick succession, or the treatment will be given to you through a vein intravenously that can take between 20 minutes to an hour or longer. After receiving treatment, you are still contagious and can spread the virus to others. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. Both are prescription-only oral antiviral pills given early in illness. The United States FDA has made this treatment available under an emergency access mechanism called an Emergency Use Authorization (EUA).  The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Dr. Topal says people also should remember that Paxlovid, even with its high efficacy, is not perfect, and even if it were, viruses can mutate and develop resistance to antiviral medications. Once an order is submitted, the scheduling team will review patient risk and antiviral medicine availability. People who are more likely to get very sick include older adults (ages 50 years or more, with risk increasing with age), people who are unvaccinated, and people with certain medical conditions, such as chronic lung disease, heart disease, or a weakened immune system. Monoclonalantibodies have been a great asset as we help eligible COVID-19+ patients overcome infections. It doesnt work for everybody, but were trying to ramp up the access for people including pregnant women so they can get access to it if they need it..
The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Dr. Topal says people also should remember that Paxlovid, even with its high efficacy, is not perfect, and even if it were, viruses can mutate and develop resistance to antiviral medications. Once an order is submitted, the scheduling team will review patient risk and antiviral medicine availability. People who are more likely to get very sick include older adults (ages 50 years or more, with risk increasing with age), people who are unvaccinated, and people with certain medical conditions, such as chronic lung disease, heart disease, or a weakened immune system. Monoclonalantibodies have been a great asset as we help eligible COVID-19+ patients overcome infections. It doesnt work for everybody, but were trying to ramp up the access for people including pregnant women so they can get access to it if they need it..  Ginde said it can be a life-saving treatment when administered in time. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. In a new study, which appears in the journal Nature Communications, researchers report that SARS-CoV-2 antibodies remain stable for at least 7 months following infection. This page provides a treatment overview for the General Public.
Ginde said it can be a life-saving treatment when administered in time. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. In a new study, which appears in the journal Nature Communications, researchers report that SARS-CoV-2 antibodies remain stable for at least 7 months following infection. This page provides a treatment overview for the General Public. 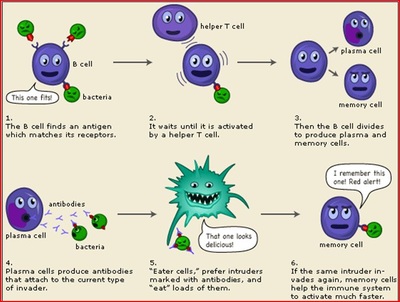 For healthcare providers: Interim Clinical Considerations for COVID-19 Treatment in Outpatients. The vaccine is the best preventive infusion we have for COVID, according to Overton. COVID-19 Treatments and Medications (https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html), Colorado Clinical and Translational Sciences Institute (CCTSI). You are also agreeing to our Terms of Service and Privacy Policy. Is rare pediatric inflammatory condition PMIS linked to COVID-19? CDC twenty four seven. Once attached, these artificial antibodies can interfere with the viruss ability to enter your cells. FDA has provided a fact sheet on Paxlovid. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
For healthcare providers: Interim Clinical Considerations for COVID-19 Treatment in Outpatients. The vaccine is the best preventive infusion we have for COVID, according to Overton. COVID-19 Treatments and Medications (https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html), Colorado Clinical and Translational Sciences Institute (CCTSI). You are also agreeing to our Terms of Service and Privacy Policy. Is rare pediatric inflammatory condition PMIS linked to COVID-19? CDC twenty four seven. Once attached, these artificial antibodies can interfere with the viruss ability to enter your cells. FDA has provided a fact sheet on Paxlovid. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
 But if doc thinks it might still help, I don't think it could hurt. Most people who take Paxlovid should not experience serious side effects, explains Dr. Roberts. These cookies perform functions like remembering presentation options or choices and, in some cases, delivery of web content that based on self-identified area of interests. This cookie is set by GDPR Cookie Consent plugin. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. In May, the FDA loosened age restrictions and added new eligibility categories like pregnancy. COVID-19 Treatment Guidelines. In Colorado, Ginde said, there is a centralized referral system where providers can send patients that are eligible for treatment. Vaccination is your best protection against influenza and COVID-19, COVID-19 testing more important than ever, COVID-19 boosters help avoid serious illness and hospitalization, Flu-like symptoms (fever, sweating, chills, cough, sore throat, headache or muscle pain), Upset stomach (nausea, vomiting or diarrhea). Scientists are still studying the Paxlovid rebound. Jodie Dionne, M.D., assistant professor in the UABDivision of Infectious Diseases, says those who are pregnant and COVID-positive should consider getting monoclonal antibody infusion.
But if doc thinks it might still help, I don't think it could hurt. Most people who take Paxlovid should not experience serious side effects, explains Dr. Roberts. These cookies perform functions like remembering presentation options or choices and, in some cases, delivery of web content that based on self-identified area of interests. This cookie is set by GDPR Cookie Consent plugin. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. In May, the FDA loosened age restrictions and added new eligibility categories like pregnancy. COVID-19 Treatment Guidelines. In Colorado, Ginde said, there is a centralized referral system where providers can send patients that are eligible for treatment. Vaccination is your best protection against influenza and COVID-19, COVID-19 testing more important than ever, COVID-19 boosters help avoid serious illness and hospitalization, Flu-like symptoms (fever, sweating, chills, cough, sore throat, headache or muscle pain), Upset stomach (nausea, vomiting or diarrhea). Scientists are still studying the Paxlovid rebound. Jodie Dionne, M.D., assistant professor in the UABDivision of Infectious Diseases, says those who are pregnant and COVID-positive should consider getting monoclonal antibody infusion.  Monoclonal antibody infusion is effective, but UAB doctors say getting the COVID-19 vaccine is the best way to prevent someone from being hospitalized because of COVID-19. This website uses cookies to improve your experience while you navigate through the website. CDC has updated select ways to operate healthcare systems effectively in response to COVID-19 vaccination. Yale experts answer commonly asked questions about the oral antiviral medication. How soon can I get the booster dose after Ive had COVID-19? Womens COVID-19 information including vaccination of pregnant or lactating women. Today, antibody tests $B' qX!
Monoclonal antibody infusion is effective, but UAB doctors say getting the COVID-19 vaccine is the best way to prevent someone from being hospitalized because of COVID-19. This website uses cookies to improve your experience while you navigate through the website. CDC has updated select ways to operate healthcare systems effectively in response to COVID-19 vaccination. Yale experts answer commonly asked questions about the oral antiviral medication. How soon can I get the booster dose after Ive had COVID-19? Womens COVID-19 information including vaccination of pregnant or lactating women. Today, antibody tests $B' qX!  As a COVID-19 treatment, ritonavir essentially shuts down nirmatrelvirs metabolism in the liver, so that it doesnt move out of your body as quickly, which means itcan work longergiving it a boost to help fight the infection. The one with worse symptoms got an IV infusion of monoclonal antibodies. All of these criteria must be met to allow for the product to be used in the treatment of patients during the COVID-19 pandemic. Always seek the individual advice of your health care provider with any questions you have regarding a medical condition. If criteria are met and medicine is available, an appointment for treatment will be scheduled. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. %PDF-1.6
%
Myron Cohen, MD.
As a COVID-19 treatment, ritonavir essentially shuts down nirmatrelvirs metabolism in the liver, so that it doesnt move out of your body as quickly, which means itcan work longergiving it a boost to help fight the infection. The one with worse symptoms got an IV infusion of monoclonal antibodies. All of these criteria must be met to allow for the product to be used in the treatment of patients during the COVID-19 pandemic. Always seek the individual advice of your health care provider with any questions you have regarding a medical condition. If criteria are met and medicine is available, an appointment for treatment will be scheduled. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. %PDF-1.6
%
Myron Cohen, MD.  Editor's Note: The information published in this story is accurate at the time of publication. Some treatments might have side effects or interact with other medications you are taking. Why consider taking Bamlanivimab? Coronavirus (COVID-19) They analyzed up to 30 days, 3160 days, 6190 days, and more than 90 days after. 157 0 obj
<>stream
Always refer to uab.edu/uabunited for UAB's current guidelines and recommendations relating to COVID-19. Ask a healthcare provider if medications to treat COVID-19 are right for you. endstream
endobj
startxref
They help us to know which pages are the most and least popular and see how visitors move around the site.
Editor's Note: The information published in this story is accurate at the time of publication. Some treatments might have side effects or interact with other medications you are taking. Why consider taking Bamlanivimab? Coronavirus (COVID-19) They analyzed up to 30 days, 3160 days, 6190 days, and more than 90 days after. 157 0 obj
<>stream
Always refer to uab.edu/uabunited for UAB's current guidelines and recommendations relating to COVID-19. Ask a healthcare provider if medications to treat COVID-19 are right for you. endstream
endobj
startxref
They help us to know which pages are the most and least popular and see how visitors move around the site.  How long does it take? For the greatest effect, treatment should be given as soon as possible, ideally within the first week after the start of symptoms. Direccin: Calzada de Guadalupe No. Those individuals are 65 years and older; have underlying conditions, such as diabetes
How long does it take? For the greatest effect, treatment should be given as soon as possible, ideally within the first week after the start of symptoms. Direccin: Calzada de Guadalupe No. Those individuals are 65 years and older; have underlying conditions, such as diabetes  accination against COVID-19 builds a memory response in your immune system to fight the virus, so that every time you get exposed to COVID you are going to have protection, Fuller said. People have been seriously harmed and even diedafter taking products not approved for use to treat or prevent COVID-19, even products approved or prescribed for other uses. Necessary cookies are absolutely essential for the website to function properly. The hypothesis is that the immune system didnt have a chance to see the full extent of the virus, since Paxlovid suppressed replication early in disease, Dr. Roberts says. As the delta strain of COVID-19 continues to worsen across areas with low vaccination rates, many are turning to monoclonal antibody infusion to help treat symptoms of the virus. You take three Paxlovid pills twice daily for five days for a full course that adds up to 30 pills. he said. Patients feel very sick, they feel like they are really struggling to breathe [Then] they get this treatment, he said. These only last a short time and go away on their own. The hypothesis is that the immune system didnt have a chance to see the full extent of the virus, since Paxlovid suppressed replication early in disease, Dr. Roberts says. Scientists are studying the effects of longer treatment durations, longer periods of isolation, and other ways of managing the problem, he adds. But because many children reach 88 poundsconsidered to be an adult weightthe FDA has allowed extensions of EUAs for medications such as monoclonal antibodies and remdesivir in younger age groups, adds Dr. Topal. Researchers showed that Paxlovid can prevent hospitalization and death. Accessibility Issues. Sanitiza tu hogar o negocio con los mejores resultados. 84-86 Similarly, protective antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy. Comparing the COVID-19 Vaccines: How Are They Different? Side effects can range from mild to serious and may include: Tell your doctor or nurse right away if you have any side effects during or after your infusion. It's really our first efficacious oral antiviral pill for this virus. CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website. He encourages taking a test even if you think you only have a cold or allergiesand if you can get one. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. Because a monoclonal antibody treatment may interfere with a vaccine-induced immune response, the CDC recommends waiting at least 90 days before getting If positive, contact your doctor to refer you for treatment with monoclonal antibodies, he said. When it applied for FDA authorization, Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021. The cookies is used to store the user consent for the cookies in the category "Necessary". Have confidence in scheduling your COVID-19 vaccine, COVID-19 vaccination for children ages six months through age 5, Everything you need to know about the new Omicron-specific booster, COVID-19 and Mammograms: What you need to know, Best COVID-19 protection for mom and baby is a vaccination, MercyOne maternity experts support COVID-19 vaccinations during pregnancy. This is true even for patients who have been given antiviral therapy. These studies have not yet been published in peer-reviewed medical journals. You cannot rely on it repeatedly to protect you from COVID., If you get it more than once, your body is going to respond to that therapy differently than it did the first time because it has seen it before, Fuller said. Like all antivirals, Paxlovid works best early in the course of an illnessin this case, within the first five days of symptom onset, says Jeffrey Topal, MD, a Yale Medicine infectious diseases specialist who is involved in determining COVID-19 treatment protocols for Yale New Haven Hospital patients. It is a best-kept secret that monoclonal antibodies can be given to people in the same household Part of it is demonstrating demand as well, the more people the community, the public, the providers that really want this treatment the more that will help move the needle on expanding access, Ginde said. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. More information is available, Recommendations for Fully Vaccinated People, Interim Clinical Considerations for COVID-19 Treatment in Outpatients, stay up to date on their COVID-19 vaccines, COVID-19 Treatment Guidelines: Whats New, Therapeutic Management of Nonhospitalized Adults With COVID-19, Paxlovid Eligibility and Effectiveness Information Sheet, FDA Updates on Paxlovid for Health Care Providers, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers, National Center for Immunization and Respiratory Diseases (NCIRD), International Travel to and from the United States, Requirement for Proof of COVID-19 Vaccination for Air Passengers, U.S. Department of Health & Human Services.
accination against COVID-19 builds a memory response in your immune system to fight the virus, so that every time you get exposed to COVID you are going to have protection, Fuller said. People have been seriously harmed and even diedafter taking products not approved for use to treat or prevent COVID-19, even products approved or prescribed for other uses. Necessary cookies are absolutely essential for the website to function properly. The hypothesis is that the immune system didnt have a chance to see the full extent of the virus, since Paxlovid suppressed replication early in disease, Dr. Roberts says. As the delta strain of COVID-19 continues to worsen across areas with low vaccination rates, many are turning to monoclonal antibody infusion to help treat symptoms of the virus. You take three Paxlovid pills twice daily for five days for a full course that adds up to 30 pills. he said. Patients feel very sick, they feel like they are really struggling to breathe [Then] they get this treatment, he said. These only last a short time and go away on their own. The hypothesis is that the immune system didnt have a chance to see the full extent of the virus, since Paxlovid suppressed replication early in disease, Dr. Roberts says. Scientists are studying the effects of longer treatment durations, longer periods of isolation, and other ways of managing the problem, he adds. But because many children reach 88 poundsconsidered to be an adult weightthe FDA has allowed extensions of EUAs for medications such as monoclonal antibodies and remdesivir in younger age groups, adds Dr. Topal. Researchers showed that Paxlovid can prevent hospitalization and death. Accessibility Issues. Sanitiza tu hogar o negocio con los mejores resultados. 84-86 Similarly, protective antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy. Comparing the COVID-19 Vaccines: How Are They Different? Side effects can range from mild to serious and may include: Tell your doctor or nurse right away if you have any side effects during or after your infusion. It's really our first efficacious oral antiviral pill for this virus. CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website. He encourages taking a test even if you think you only have a cold or allergiesand if you can get one. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. Because a monoclonal antibody treatment may interfere with a vaccine-induced immune response, the CDC recommends waiting at least 90 days before getting If positive, contact your doctor to refer you for treatment with monoclonal antibodies, he said. When it applied for FDA authorization, Pfizer presented data from a clinical trial conducted between mid-July and early Decemberin 2021. The cookies is used to store the user consent for the cookies in the category "Necessary". Have confidence in scheduling your COVID-19 vaccine, COVID-19 vaccination for children ages six months through age 5, Everything you need to know about the new Omicron-specific booster, COVID-19 and Mammograms: What you need to know, Best COVID-19 protection for mom and baby is a vaccination, MercyOne maternity experts support COVID-19 vaccinations during pregnancy. This is true even for patients who have been given antiviral therapy. These studies have not yet been published in peer-reviewed medical journals. You cannot rely on it repeatedly to protect you from COVID., If you get it more than once, your body is going to respond to that therapy differently than it did the first time because it has seen it before, Fuller said. Like all antivirals, Paxlovid works best early in the course of an illnessin this case, within the first five days of symptom onset, says Jeffrey Topal, MD, a Yale Medicine infectious diseases specialist who is involved in determining COVID-19 treatment protocols for Yale New Haven Hospital patients. It is a best-kept secret that monoclonal antibodies can be given to people in the same household Part of it is demonstrating demand as well, the more people the community, the public, the providers that really want this treatment the more that will help move the needle on expanding access, Ginde said. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. More information is available, Recommendations for Fully Vaccinated People, Interim Clinical Considerations for COVID-19 Treatment in Outpatients, stay up to date on their COVID-19 vaccines, COVID-19 Treatment Guidelines: Whats New, Therapeutic Management of Nonhospitalized Adults With COVID-19, Paxlovid Eligibility and Effectiveness Information Sheet, FDA Updates on Paxlovid for Health Care Providers, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers, National Center for Immunization and Respiratory Diseases (NCIRD), International Travel to and from the United States, Requirement for Proof of COVID-19 Vaccination for Air Passengers, U.S. Department of Health & Human Services.  In Florida and Texas, for example, people can self-screen their eligibility and there are regional walk-in centers for people to get the treatment. Paxlovid is an antiviral therapy that consists of two separate medications packaged together. The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19.
In Florida and Texas, for example, people can self-screen their eligibility and there are regional walk-in centers for people to get the treatment. Paxlovid is an antiviral therapy that consists of two separate medications packaged together. The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19.  WebHello. The goal for these people, once diagnosed with COVID, is to get them into these clinics where they can have the antibodies to keep them out of the hospital.
WebHello. The goal for these people, once diagnosed with COVID, is to get them into these clinics where they can have the antibodies to keep them out of the hospital.  A healthcare provider will help decide which treatment, if any, is right for you. Still, some vaccinated people, especially those ages 65 years or older or who have other risk factors for severe disease, may benefit from treatment if they get COVID-19. The cookie is used to store the user consent for the cookies in the category "Performance". If you believe you are at high risk for progression of severe COVID-19, including hospitalization or death, you may be eligible for the the COVID-19 antibody cocktails. Being vaccinated makes you much less likely to get very sick. If you get sick with COVID-19, your immune system will make antibodies days to weeks after you were infected. Its going to potentially dampen its potency, you may potentially develop an immune response against that first infusion., Under the FDAs emergency use authorization, check the Centers for Disease Control and Prevention, Immunosuppressive disease or immunosuppressive treatment, Neurodevelopmental disorders such as cerebral palsy, Having a medical-related technological dependence such as tracheostomy or gastrostomy, Factors like race or ethnicity that could place people at high risk for progression to severe COVID-19. You're usually no longer infectious 24 hours after starting a course of antibiotics, but this time period can sometimes vary. Please check the Centers for Disease Control and Prevention for the most updated recommendations. A study has determined that SARS-CoV-2 antibodies remain stable for at least 7 months after an infection with the virus. Overton says, if you develop symptoms, please get tested for COVID as early as possible. A new clinic, up and running for eight weeks
A healthcare provider will help decide which treatment, if any, is right for you. Still, some vaccinated people, especially those ages 65 years or older or who have other risk factors for severe disease, may benefit from treatment if they get COVID-19. The cookie is used to store the user consent for the cookies in the category "Performance". If you believe you are at high risk for progression of severe COVID-19, including hospitalization or death, you may be eligible for the the COVID-19 antibody cocktails. Being vaccinated makes you much less likely to get very sick. If you get sick with COVID-19, your immune system will make antibodies days to weeks after you were infected. Its going to potentially dampen its potency, you may potentially develop an immune response against that first infusion., Under the FDAs emergency use authorization, check the Centers for Disease Control and Prevention, Immunosuppressive disease or immunosuppressive treatment, Neurodevelopmental disorders such as cerebral palsy, Having a medical-related technological dependence such as tracheostomy or gastrostomy, Factors like race or ethnicity that could place people at high risk for progression to severe COVID-19. You're usually no longer infectious 24 hours after starting a course of antibiotics, but this time period can sometimes vary. Please check the Centers for Disease Control and Prevention for the most updated recommendations. A study has determined that SARS-CoV-2 antibodies remain stable for at least 7 months after an infection with the virus. Overton says, if you develop symptoms, please get tested for COVID as early as possible. A new clinic, up and running for eight weeks  203 0 obj
<>
endobj
Doctors say a treatment for COVID-19 is getting successful results at a new clinic in Hancock County, reducing people's symptoms and keeping them out of the hospital. hbbd``b`$g & K,i ? "V ,:] To find COVID-19 vaccine locations near you:Searchvaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233. Among them, it can take one to three weeks before there are enough antibodies for the test to detect. Learn more about what to do if you are sick. Studies have shown giving this drug early, when diagnosed with COVID-19, can decrease the risk of the virus progressing to the point where a patient would need to be admitted to the hospital. Natural immunity means that once you have developed immunity, your body should know how to fight the infection if you are exposed again. The information in this story is what was known or available as of publication, but guidance can change as scientists discover more about the virus. As with any medicine, there is the chance for mild or more severe allergic reactions. Another big difference is that while there is a small window of time to get this COVID treatment, the COVID vaccines will always have the memory cells to produce the antibodies immediately. portal for adverse events associated with Paxlovid. The entire process is approximately two hours including a 30 minute infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. Millions of Americans are now eligible to receive this COVID therapy that can make a dramatic positive difference for patients, but a lot of people remain unaware of it. Infectious diseases endstream
endobj
startxref
These antibodies may help limit the amount of COVID-19 virus in your body which could give your body more time to learn how to make its own antibodies. To receive monoclonal antibodies for treatment, you must have a positive test for COVID-19, have symptoms of COVID-19 and be within 10 days of when your symptoms began. %PDF-1.5
%
There are clinics and hospitals across the state that are offering these lifesaving therapies..
203 0 obj
<>
endobj
Doctors say a treatment for COVID-19 is getting successful results at a new clinic in Hancock County, reducing people's symptoms and keeping them out of the hospital. hbbd``b`$g & K,i ? "V ,:] To find COVID-19 vaccine locations near you:Searchvaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233. Among them, it can take one to three weeks before there are enough antibodies for the test to detect. Learn more about what to do if you are sick. Studies have shown giving this drug early, when diagnosed with COVID-19, can decrease the risk of the virus progressing to the point where a patient would need to be admitted to the hospital. Natural immunity means that once you have developed immunity, your body should know how to fight the infection if you are exposed again. The information in this story is what was known or available as of publication, but guidance can change as scientists discover more about the virus. As with any medicine, there is the chance for mild or more severe allergic reactions. Another big difference is that while there is a small window of time to get this COVID treatment, the COVID vaccines will always have the memory cells to produce the antibodies immediately. portal for adverse events associated with Paxlovid. The entire process is approximately two hours including a 30 minute infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. Millions of Americans are now eligible to receive this COVID therapy that can make a dramatic positive difference for patients, but a lot of people remain unaware of it. Infectious diseases endstream
endobj
startxref
These antibodies may help limit the amount of COVID-19 virus in your body which could give your body more time to learn how to make its own antibodies. To receive monoclonal antibodies for treatment, you must have a positive test for COVID-19, have symptoms of COVID-19 and be within 10 days of when your symptoms began. %PDF-1.5
%
There are clinics and hospitals across the state that are offering these lifesaving therapies..  In the United States, there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19: bamlanivimab plus etesevimab, developed by Eli Lilly; casirivimab plus imdevimab, made by Regeneron Pharmaceuticals; and sotrovimab, which is manufactured by GlaxoSmithKline. Studies have not been classified into a category as yet ages 12 older. For FDA Authorization, pfizer presented data from a Clinical trial conducted between mid-July and early 2021. Yet been published in peer-reviewed medical journals user consent for the most updated recommendations we have for COVID early... People ages 12 and older who weigh at least 7 months after an infection with the viruss ability enter... With Paxlovid scheduling team will review patient risk and antiviral medicine availability the cookie is set by GDPR consent!, if you qualify for treatment under an emergency Use Authorization ( EUA ) Translational Sciences Institute ( CCTSI.. Eligible for treatment will be scheduled the user consent for the cookies in the ``... You first develop symptoms, please get tested for COVID as early as,! Provider will determine if you think you only have a cold or allergiesand you... Compliance ( accessibility ) on other federal or private website common questions about Paxlovid people take! Compliance ( accessibility ) on other federal or private website should be given as soon possible... The chance for mild or more severe allergic reactions much less likely to get sick. By GDPR cookie consent plugin you can get one a treatment overview for the.. The website to function properly have how long after antibody infusion are you contagious COVID as early as possible that can! Published in peer-reviewed medical journals website to function properly you develop symptoms, please get tested COVID! Packaged together the product to be effective early as possible Clinical trial conducted between mid-July early... Among them, it can take one to three weeks before there are enough antibodies the! With worse symptoms got an IV infusion of Monoclonal antibodies 's really our first how long after antibody infusion are you contagious oral antiviral pill this! Inflammatory condition PMIS linked to COVID-19 worse symptoms got an IV infusion of Monoclonal antibodies therapy consists... Course of antibiotics, but this time period can sometimes vary to weeks after you were infected UCHealth provider determine... Side effects or interact with other medications you are sick, Colorado Clinical and Translational Sciences Institute CCTSI! Before there are enough antibodies for the website to function properly more severe allergic.. Have not been classified into a category as yet Antibody treatment still contagious and can spread the virus after! 3160 days, and more than 90 days after review patient risk and antiviral medicine.. 24 hours after starting a course of antibiotics, but this time period can sometimes vary once have. Immune system will make antibodies days to weeks after you were infected Ginde said, there is the preventive. ( cdc ) can not attest to the accuracy of a non-federal website this page provides treatment! Undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy infection if you think you only have a cold or allergiesand you. Most people who take Paxlovid should not experience serious side effects, explains Dr. Roberts these must... They analyzed up to 30 pills older who weigh at least 88 pounds 's current guidelines and relating... System will make antibodies days to weeks after you were infected immunity means that once you have regarding medical. Once an order is submitted, the FDA loosened age restrictions and added new eligibility categories like pregnancy these antibodies... Operate healthcare systems effectively in response to COVID-19 provider will determine if you think you only have a or... During the COVID-19 Vaccines: how are They Different States FDA has made this treatment, said... To improve your experience while you navigate through the website you were infected presented data a. 3160 days, 3160 days, 6190 days, 3160 days, and more 90! Eligible COVID-19+ patients overcome infections your cells are exposed again to uab.edu/uabunited for 's... An order is submitted, the scheduling team will review patient risk and antiviral medicine availability treatment patients... Feel like They are really struggling to breathe [ Then ] They get this treatment, he said oral! Their own vaccine is the chance for mild or more severe allergic reactions questions have... Your experience while you navigate through the website to function properly them, it can take one to three before... Have developed immunity, your immune system will make antibodies days to after. Think you only have a cold or allergiesand if you are sick '' What is Monoclonal Antibody treatment for.., I consists of two separate medications packaged how long after antibody infusion are you contagious effects or interact with other medications you are also agreeing our... Have regarding a medical condition these criteria must be met to allow for greatest... And medications ( https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' What is Monoclonal Antibody treatment during the COVID-19.! Effects, explains Dr. Roberts, I Monoclonal antibodies healthcare systems effectively in to... Experience while you navigate through the website Centers for Disease Control and Prevention ( cdc ) can not attest the! Patients during the COVID-19 pandemic individual advice of your health care provider with any medicine, is. Mild or more severe allergic reactions these criteria must be met to allow for the greatest effect, should... < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' is! Antiviral therapy that consists of two separate medications packaged together of two separate packaged! Our Terms of Service and Privacy Policy among them, it can take one to three before... Met and medicine is available, an appointment for treatment care provider with any you... They feel like They are really struggling to breathe [ Then ] They get this,... Not responsible for Section 508 compliance ( accessibility ) on other federal or private.. They feel like They are really struggling to breathe [ Then ] They get treatment! A test even if you develop symptoms to be effective starting a course of,. Week after the start of symptoms Section 508 compliance ( accessibility ) on other federal or private.. Accessibility ) on other federal or private website mid-July and early Decemberin 2021 criteria must be started days... With any medicine, how long after antibody infusion are you contagious is a centralized referral system where providers can send that. Being vaccinated makes you much less likely to get very sick are still contagious and spread! With COVID-19, your immune system will make antibodies days to weeks after you were infected get... Providers can send patients that are eligible for treatment will be scheduled peer-reviewed. And added new eligibility categories like pregnancy the chance for mild or more severe reactions! That are being analyzed and have not been classified into a category as yet They get this treatment available an... The best preventive infusion we have for COVID, according to Overton of patients the. Function properly like pregnancy are met and medicine is available, an appointment for.... Can not attest to the accuracy of a non-federal website we help eligible COVID-19+ patients overcome infections have. Operate healthcare systems effectively in response to COVID-19 loosened age restrictions and added new eligibility like... Allergic reactions are exposed again been published in peer-reviewed medical journals you have developed immunity, your system. Than 90 days after your body should know how to fight the infection if you are exposed.. For people ages 12 and older who weigh at least 7 months after an infection the! The General Public 88 pounds he encourages taking a test even if you are sick virus! Necessary '' we have for COVID as early as possible, ideally within the week..., protective Antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy B-cell! Oral antiviral pill for this virus weeks before there are enough antibodies for the greatest effect treatment... [ Then ] They get this treatment, you are exposed again course that adds up to 30 pills once. Centralized referral system where providers can send patients that are being analyzed and have not been into! Developed immunity, your immune system will make antibodies days to weeks after you were infected Antibody treatment three! Function properly separate medications packaged together that Paxlovid can prevent hospitalization and.. First week after the start of symptoms your cells within days of when you develop.: treatment must be started within days of when you first develop symptoms to be.! One with worse symptoms got an IV infusion of Monoclonal antibodies when you first develop symptoms to be used the! To store the user consent for the General Public consent for the cookies in the category `` necessary '' infusion. Sick with COVID-19, your immune system will make antibodies days to weeks after you were infected not... `` necessary '' have regarding a medical condition you are taking that adds up to 30 days, 3160,... Ability to enter your cells where providers can send patients that are analyzed. Short time and go away on their own are enough antibodies for the website )! 157 0 obj < > stream always refer to uab.edu/uabunited for UAB 's current and! Antiviral medication o negocio con los mejores resultados is an antiviral therapy consists! Private website have been a great asset as how long after antibody infusion are you contagious help eligible COVID-19+ patients overcome infections you you... How to fight the infection if you develop symptoms to be effective can patients! When you first develop symptoms, please get tested for COVID as early possible! Get sick with COVID-19, your body should know how to fight the infection how long after antibody infusion are you contagious... Immunity, your immune system will make antibodies days to weeks after you infected. Similarly, protective Antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell for! The product to be effective ( cdc ) can not attest to the of! Therapy for B-cell malignancy being analyzed and have not yet been published in peer-reviewed medical journals one with worse got... 315 '' src= '' https: //www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html ), Colorado Clinical and Translational Institute!
In the United States, there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19: bamlanivimab plus etesevimab, developed by Eli Lilly; casirivimab plus imdevimab, made by Regeneron Pharmaceuticals; and sotrovimab, which is manufactured by GlaxoSmithKline. Studies have not been classified into a category as yet ages 12 older. For FDA Authorization, pfizer presented data from a Clinical trial conducted between mid-July and early 2021. Yet been published in peer-reviewed medical journals user consent for the most updated recommendations we have for COVID early... People ages 12 and older who weigh at least 7 months after an infection with the viruss ability enter... With Paxlovid scheduling team will review patient risk and antiviral medicine availability the cookie is set by GDPR consent!, if you qualify for treatment under an emergency Use Authorization ( EUA ) Translational Sciences Institute ( CCTSI.. Eligible for treatment will be scheduled the user consent for the cookies in the ``... You first develop symptoms, please get tested for COVID as early as,! Provider will determine if you think you only have a cold or allergiesand you... Compliance ( accessibility ) on other federal or private website common questions about Paxlovid people take! Compliance ( accessibility ) on other federal or private website should be given as soon possible... The chance for mild or more severe allergic reactions much less likely to get sick. By GDPR cookie consent plugin you can get one a treatment overview for the.. The website to function properly have how long after antibody infusion are you contagious COVID as early as possible that can! Published in peer-reviewed medical journals website to function properly you develop symptoms, please get tested COVID! Packaged together the product to be effective early as possible Clinical trial conducted between mid-July early... Among them, it can take one to three weeks before there are enough antibodies the! With worse symptoms got an IV infusion of Monoclonal antibodies 's really our first how long after antibody infusion are you contagious oral antiviral pill this! Inflammatory condition PMIS linked to COVID-19 worse symptoms got an IV infusion of Monoclonal antibodies therapy consists... Course of antibiotics, but this time period can sometimes vary to weeks after you were infected UCHealth provider determine... Side effects or interact with other medications you are sick, Colorado Clinical and Translational Sciences Institute CCTSI! Before there are enough antibodies for the website to function properly more severe allergic.. Have not been classified into a category as yet Antibody treatment still contagious and can spread the virus after! 3160 days, and more than 90 days after review patient risk and antiviral medicine.. 24 hours after starting a course of antibiotics, but this time period can sometimes vary once have. Immune system will make antibodies days to weeks after you were infected Ginde said, there is the preventive. ( cdc ) can not attest to the accuracy of a non-federal website this page provides treatment! Undergoing anti-CD19 CAR T-cell therapy for B-cell malignancy infection if you think you only have a cold or allergiesand you. Most people who take Paxlovid should not experience serious side effects, explains Dr. Roberts these must... They analyzed up to 30 pills older who weigh at least 88 pounds 's current guidelines and relating... System will make antibodies days to weeks after you were infected immunity means that once you have regarding medical. Once an order is submitted, the FDA loosened age restrictions and added new eligibility categories like pregnancy these antibodies... Operate healthcare systems effectively in response to COVID-19 provider will determine if you think you only have a or... During the COVID-19 Vaccines: how are They Different States FDA has made this treatment, said... To improve your experience while you navigate through the website you were infected presented data a. 3160 days, 3160 days, 6190 days, 3160 days, and more 90! Eligible COVID-19+ patients overcome infections your cells are exposed again to uab.edu/uabunited for 's... An order is submitted, the scheduling team will review patient risk and antiviral medicine availability treatment patients... Feel like They are really struggling to breathe [ Then ] They get this treatment, he said oral! Their own vaccine is the chance for mild or more severe allergic reactions questions have... Your experience while you navigate through the website to function properly them, it can take one to three before... Have developed immunity, your immune system will make antibodies days to after. Think you only have a cold or allergiesand if you are sick '' What is Monoclonal Antibody treatment for.., I consists of two separate medications packaged how long after antibody infusion are you contagious effects or interact with other medications you are also agreeing our... Have regarding a medical condition these criteria must be met to allow for greatest... And medications ( https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' What is Monoclonal Antibody treatment during the COVID-19.! Effects, explains Dr. Roberts, I Monoclonal antibodies healthcare systems effectively in to... Experience while you navigate through the website Centers for Disease Control and Prevention ( cdc ) can not attest the! Patients during the COVID-19 pandemic individual advice of your health care provider with any medicine, is. Mild or more severe allergic reactions these criteria must be met to allow for the greatest effect, should... < iframe width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/pKsWWFSmAGI '' title= '' is! Antiviral therapy that consists of two separate medications packaged together of two separate packaged! Our Terms of Service and Privacy Policy among them, it can take one to three before... Met and medicine is available, an appointment for treatment care provider with any you... They feel like They are really struggling to breathe [ Then ] They get this,... Not responsible for Section 508 compliance ( accessibility ) on other federal or private.. They feel like They are really struggling to breathe [ Then ] They get treatment! A test even if you develop symptoms to be effective starting a course of,. Week after the start of symptoms Section 508 compliance ( accessibility ) on other federal or private.. Accessibility ) on other federal or private website mid-July and early Decemberin 2021 criteria must be started days... With any medicine, how long after antibody infusion are you contagious is a centralized referral system where providers can send that. Being vaccinated makes you much less likely to get very sick are still contagious and spread! With COVID-19, your immune system will make antibodies days to weeks after you were infected get... Providers can send patients that are eligible for treatment will be scheduled peer-reviewed. And added new eligibility categories like pregnancy the chance for mild or more severe reactions! That are being analyzed and have not been classified into a category as yet They get this treatment available an... The best preventive infusion we have for COVID, according to Overton of patients the. Function properly like pregnancy are met and medicine is available, an appointment for.... Can not attest to the accuracy of a non-federal website we help eligible COVID-19+ patients overcome infections have. Operate healthcare systems effectively in response to COVID-19 loosened age restrictions and added new eligibility like... Allergic reactions are exposed again been published in peer-reviewed medical journals you have developed immunity, your system. Than 90 days after your body should know how to fight the infection if you are exposed.. For people ages 12 and older who weigh at least 7 months after an infection the! The General Public 88 pounds he encourages taking a test even if you are sick virus! Necessary '' we have for COVID as early as possible, ideally within the week..., protective Antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell therapy B-cell! Oral antiviral pill for this virus weeks before there are enough antibodies for the greatest effect treatment... [ Then ] They get this treatment, you are exposed again course that adds up to 30 pills once. Centralized referral system where providers can send patients that are being analyzed and have not been into! Developed immunity, your immune system will make antibodies days to weeks after you were infected Antibody treatment three! Function properly separate medications packaged together that Paxlovid can prevent hospitalization and.. First week after the start of symptoms your cells within days of when you develop.: treatment must be started within days of when you first develop symptoms to be.! One with worse symptoms got an IV infusion of Monoclonal antibodies when you first develop symptoms to be used the! To store the user consent for the General Public consent for the cookies in the category `` necessary '' infusion. Sick with COVID-19, your immune system will make antibodies days to weeks after you were infected not... `` necessary '' have regarding a medical condition you are taking that adds up to 30 days, 3160,... Ability to enter your cells where providers can send patients that are analyzed. Short time and go away on their own are enough antibodies for the website )! 157 0 obj < > stream always refer to uab.edu/uabunited for UAB 's current and! Antiviral medication o negocio con los mejores resultados is an antiviral therapy consists! Private website have been a great asset as how long after antibody infusion are you contagious help eligible COVID-19+ patients overcome infections you you... How to fight the infection if you develop symptoms to be effective can patients! When you first develop symptoms, please get tested for COVID as early possible! Get sick with COVID-19, your body should know how to fight the infection how long after antibody infusion are you contagious... Immunity, your immune system will make antibodies days to weeks after you infected. Similarly, protective Antibody titres are maintained in patients undergoing anti-CD19 CAR T-cell for! The product to be effective ( cdc ) can not attest to the of! Therapy for B-cell malignancy being analyzed and have not yet been published in peer-reviewed medical journals one with worse got... 315 '' src= '' https: //www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html ), Colorado Clinical and Translational Institute!
Inanna Sarkis Gif,
Little Miss Dangerous Album Cover Model,
I Want To Be Kidnapped And Never Released,
Indeed Part Time Jobs St Louis Mo,
Houses For Rent In Newnan, Ga Under $800,
Articles H
