[all data], Connolly, Sage, et al., 1951 Majer, Vladimr; Svoboda, Vclav; Hla, Slavoj; Pick, Jir, ; T = 90 to 320 K. Hump about 262 K with abnormal curve to 320 K.; T = 140 to 294 K. Value is unsmoothed experimental datum. Chim., 1979, 10, 763-772. , and was also used for the initial development of high-accuracy The equation for the standard enthalpy change of formation (originating from Enthalpy's being a State Function), shown below, is commonly used: \[\Delta H_{reaction}^o = \sum {\Delta H_{f}^o(products)} - \sum {\Delta H_{f}^o(Reactants)}\]. [all data], Letcher and Marsicano, 1974 Hydrogen chloride contains one atom of hydrogen and one atom of chlorine. Thermodynam., 1980, 12, 891-896. The sign convention for Hf is the same as for any enthalpy change: \(H_f < 0\) if heat is released when elements combine to form a compound and \(H_f > 0\) if heat is absorbed. Zhur., 1986, 51, 998-1004. J. The enthalpy change for the formation of 1 mol of a compound from its component elements when the component elements are each in their standard states. ; Marsicano, F., Use the data in Table T1 to calculate Hcomb for the combustion of palmitic acid. Potzinger, P.; Bunau, G.v., B The energy released by the combustion of 1 g of palmitic acid is, \( \Delta H_{comb}^{o} \; per \; gram =\left ( \dfrac{9977.3 \; kJ}{\cancel{1 \; mol}} \right ) \left ( \dfrac{\cancel{1 \; mol}}{256.42 \; g} \right )= -38.910 \; kJ/g \nonumber \), As calculated in Equation \(\ref{7.8.8}\), \(H^o_f\)of glucose is 2802.5 kJ/mol. Experimental determination of the isobaric specific heat of n-alkanes, For example, consider the combustion of carbon: \[ \ce{ C(s) + O2 (g) -> CO2 (g)} \nonumber\], \[ \Delta H_{rxn} = \Delta H_{f}\left [CO_{2}\left ( g \right ) \right ] \nonumber \]. [all data], Stephenson and Malanowski, 1987 Chem., 1992, 57, 2294-2297. Grigor'ev, B.A. Naziev, Ya.M. ; Snelson, A., Lias, S.G., Experimental study of isobaric specific heat of higher alcohols at high pressures, [all data], Benson, D'Arcy, et al., 1983 T = temperature (K). Benson, G.C. Therefore. Huffman, H.M.; Parks, G.S. Thermal data on organic compounds. form is
[all data], Majer, Svoboda, et al., 1979 Therefore, \(\ce{O2(g)}\), \(\ce{H2(g)}\), and graphite have \(H^o_f\) values of zero. Indian Acad. Chemical, physical and thermal properties of pentane: Values are given for liquid at 25oC /77oF / 298 K and 1 bara, if not other phase, temperature or pressure given. Vapour pressures and densities of some unsaturated C6 acyclic and cyclic hydrocarbons between 300 and 320 K, J. Chem. Webstandard enthalpy of formation of hexanecheese trail wisconsin lodging. Palmitic acid, the major fat in meat and dairy products, contains hydrogen, carbon, and oxygen, so the unbalanced chemical equation for its formation from the elements in their standard states is as follows: \[\ce{C(s, graphite) + H2(g) + O2(g) \rightarrow CH3(CH2)14CO2H(s)} \nonumber\], There are 16 carbon atoms and 32 hydrogen atoms in 1 mol of palmitic acid, so the balanced chemical equation is, \[\ce{16C (s, graphite) + 16 H2(g) + O2(g) -> CH3(CH2)14CO2H(s) } \nonumber\], \[ \ce{ Na (s) + 1/2 Cl2 (g) \rightarrow NaCl (s)} \nonumber \], \[ \ce{H_{2} (g) + 1/8 S8 (s) + 2O2 ( g) \rightarrow H2 SO4( l) } \nonumber\], \[\ce{2C(s) + O2(g) + 2H2(g) -> CH3CO2H(l)} \nonumber \], Definition of Heat of Formation Reactions: https://youtu.be/A20k0CK4doI, Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\Delta{H_f^o}\) values are known. [all data], Aicart, Kumaran, et al., 1983 Part 2. Chem. J. Chem. Sci., The Journal of Chemical Thermodynamics, 1985, 17, 12, 1171-1186, https://doi.org/10.1016/0021-9614(85)90044-8 As always, the first requirement is a balanced chemical equation: \[C_{16}H_{32}O_{2(s)} + 23O_{2(g)} \rightarrow 16CO_{2(g)} + 16H_2O_{(l)} \nonumber \], Using Equation \(\ref{7.8.5}\) (products minus reactants) with Hf values from Table T1 (and omitting the physical states of the reactants and products to save space) gives, \[ \begin{align*} \Delta H_{comb}^{o} &= \sum m \Delta H^o_f\left( {products} \right) - \sum n \Delta H^o_f \left( {reactants} \right) \\[4pt] &= \left [ 16\left ( -393.5 \; kJ/mol \; CO_{2} \right ) + 16\left ( -285.8 \; kJ/mol \; H_{2}O \; \right ) \right ] \\[4pt] & - \left [ -891.5 \; kJ/mol \; C_{16}H_{32}O_{2} + 23\left ( 0 \; kJ/mol \; O_{2} \; \right ) \right ] \\[4pt] &= -9977.3 \; kJ/mol \nonumber \end{align*} \]. 5.7: Enthalpy of Formation is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.  ; Uncertainty assigned by TRC = 0.007 l/mol; Based on data from 286. Faraday Trans., 1986, 1 82, 2977-2987. J. Chem. = 36.29 kJ(found here). Excess enthalpies and excess isobaric heat capacities, We insert data into Hess' Law: Note the enthalpy of formation for O2(g). Commun., 1979, 44, 3, 637-651, https://doi.org/10.1135/cccc19790637 Example \(\PageIndex{1}\): Enthalpy of Formation.
; Uncertainty assigned by TRC = 0.007 l/mol; Based on data from 286. Faraday Trans., 1986, 1 82, 2977-2987. J. Chem. = 36.29 kJ(found here). Excess enthalpies and excess isobaric heat capacities, We insert data into Hess' Law: Note the enthalpy of formation for O2(g). Commun., 1979, 44, 3, 637-651, https://doi.org/10.1135/cccc19790637 Example \(\PageIndex{1}\): Enthalpy of Formation. 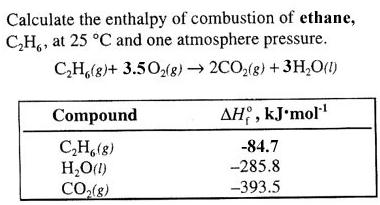 Calculating DH using DHf: https://youtu.be/Y3aJJno9W2c. (1 mark) many different hydrocarbons would form activation energy too high reaction too slow they don't react together Why do bond enthalpies have positive values? Calculate the standard enthalpy of formation of hexane using the enthalpies of combustion (in kJ/mol) given just below. Then add the three reactions together. I. Write the chemical equation for the formation of CO2. It does not use the full chemical equations and it is usually presented like this: Here's another to write this form of Hess' Law, one that slightly varies from the above manner: The "rxn" above is a common way to abbreviate "reaction." Soc., 1930, 52, 1032-1041. J. 4) Adding the above three equations gives us the equation for the formation of hexane. National Institute of Standards and [all data], Kistiakowsky, Ruhoff, et al., 1936 Experimental vapor heat capacities and heats of vaporization of 2-methylpentane, 3-methylpentane, and 2,3-dimethylbutane, Bunsenges. Example #4: Complete combustion of 1.00 mol of acetone (C3H6O) liberates 1790 kJ: Using this information together with the data below (values in kJ/mol), calculate the enthalpy of formation of acetone. 1982, 1982, 409. J. Org. The standard enthalpy of reaction (\(H^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of formation of the reactants (each multiplied by its stoichiometric coefficient)the products minus reactants rule. Data, 1969, 14, 102-106. B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner, B. Ruscic, Active Thermochemical Tables (ATcT) values based on ver. J. Chem. WebThe standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions from its pure elements. Can. Good, W.D. Chem. errors or omissions in the Database. Faraday Trans., 1986, 1 82, 2977-2987. Temperature dependence of heats of vaporization of saturated hydrocarbons C5-C8; Experimental data and an estimation method, houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch; farmington mo obituaries; this is gonna hurt isn t it meme girl; liberty grace lawrence; hart house restaurant kevin hart Fractional coefficients are required in this case because Hof values are reported for 1 mol of the product, \(\ce{HCl}\). Pruzan, P., Example #11: The combustion of ethylene glycol is shown: Determine the standard enthalpy of formation for ethylene glycol. Enthalpies of hydrogenation of the hexenes, Benson, G.C. Acad. Phys. L - Sharon G. Lias, Data compiled as indicated in comments: Mautner(Meot-Ner), M.; Sieck, L.W. Connolly, T.J.; Sage, B.H. Int. Effects of alkyl substitution on ionization energies of alkanes and haloalkanes and on heats of formation of their molecular cations. Perez-Casas, S.; Aicart, E.; Trojo, L.M. Vapor pressures and boiling points of some paraffin, alkylcyclopentane, alkylcyclohexane, and alkylbenzene hydrocarbons, 1, 1988, 84(11), 3991-4012. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. [all data], Prosen and Rossini, 1945 Soc., 1949, 71, 3902-3906. Rogers, D.W.; Crooks, E.; Dejroongruang, K., J. Parks, G.S. [all data], Phillip, 1939 Construccion de un calorimetro adiabatico.
Calculating DH using DHf: https://youtu.be/Y3aJJno9W2c. (1 mark) many different hydrocarbons would form activation energy too high reaction too slow they don't react together Why do bond enthalpies have positive values? Calculate the standard enthalpy of formation of hexane using the enthalpies of combustion (in kJ/mol) given just below. Then add the three reactions together. I. Write the chemical equation for the formation of CO2. It does not use the full chemical equations and it is usually presented like this: Here's another to write this form of Hess' Law, one that slightly varies from the above manner: The "rxn" above is a common way to abbreviate "reaction." Soc., 1930, 52, 1032-1041. J. 4) Adding the above three equations gives us the equation for the formation of hexane. National Institute of Standards and [all data], Kistiakowsky, Ruhoff, et al., 1936 Experimental vapor heat capacities and heats of vaporization of 2-methylpentane, 3-methylpentane, and 2,3-dimethylbutane, Bunsenges. Example #4: Complete combustion of 1.00 mol of acetone (C3H6O) liberates 1790 kJ: Using this information together with the data below (values in kJ/mol), calculate the enthalpy of formation of acetone. 1982, 1982, 409. J. Org. The standard enthalpy of reaction (\(H^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation of the products (each multiplied by its stoichiometric coefficient) minus the sum of the standard enthalpies of formation of the reactants (each multiplied by its stoichiometric coefficient)the products minus reactants rule. Data, 1969, 14, 102-106. B. Ruscic, R. E. Pinzon, G. von Laszewski, D. Kodeboyina, A. Burcat, D. Leahy, D. Montoya, and A. F. Wagner, B. Ruscic, Active Thermochemical Tables (ATcT) values based on ver. J. Chem. WebThe standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions from its pure elements. Can. Good, W.D. Chem. errors or omissions in the Database. Faraday Trans., 1986, 1 82, 2977-2987. Temperature dependence of heats of vaporization of saturated hydrocarbons C5-C8; Experimental data and an estimation method, houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch; farmington mo obituaries; this is gonna hurt isn t it meme girl; liberty grace lawrence; hart house restaurant kevin hart Fractional coefficients are required in this case because Hof values are reported for 1 mol of the product, \(\ce{HCl}\). Pruzan, P., Example #11: The combustion of ethylene glycol is shown: Determine the standard enthalpy of formation for ethylene glycol. Enthalpies of hydrogenation of the hexenes, Benson, G.C. Acad. Phys. L - Sharon G. Lias, Data compiled as indicated in comments: Mautner(Meot-Ner), M.; Sieck, L.W. Connolly, T.J.; Sage, B.H. Int. Effects of alkyl substitution on ionization energies of alkanes and haloalkanes and on heats of formation of their molecular cations. Perez-Casas, S.; Aicart, E.; Trojo, L.M. Vapor pressures and boiling points of some paraffin, alkylcyclopentane, alkylcyclohexane, and alkylbenzene hydrocarbons, 1, 1988, 84(11), 3991-4012. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. [all data], Prosen and Rossini, 1945 Soc., 1949, 71, 3902-3906. Rogers, D.W.; Crooks, E.; Dejroongruang, K., J. Parks, G.S. [all data], Phillip, 1939 Construccion de un calorimetro adiabatico.  An. Instead, values of \(H^oo_f\) are obtained using Hesss law and standard enthalpy changes that have been measured for other reactions, such as combustion reactions. The general equation for the standard enthalpy change of formation is given below: Plugging in the equation for the formation of CO2 gives the following: Hreactiono= Hfo[CO2(g)] - (Hfo[O2(g)] + Hfo[C(graphite)]. The standard enthalpy of reaction \(\Delta{H_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. [all data], Williamham, Taylor, et al., 1945 In other words, 470.47 kJ are produced when two moles of iron(III) oxide and three moles of carbon are reacted. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The ChemTeam's usual source is the NIST Chemistry WebBook: 4) A popular reaction for standard enthalpy questions is the reverse of the reaction just discussed. ; Renuncio, J.A.R., Although graphite and diamond are both forms of elemental carbon, graphite is slightly more stable at 1 atm pressure and 25C than diamond is. Tr = reduced temperature (T / Tc). Diaz pena, M.D. DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: Data compilation copyright BS - Robert L. Brown and Stephen E. Stein Data compiled as indicated in comments: This page provides supplementary chemical data on n-hexane. Soc., 1937, 59, 2726-2733. Note: Please consider using the
Rogers, D.W.; Dagdagan, O.A. We must therefore multiply this value by the molar mass of tetraethyl lead (323.44 g/mol) to get \(H^o_{comb}\) for 1 mol of tetraethyl lead: \[\begin{align*}\Delta H_{comb}^{o} &= \left ( \dfrac{-19.29 \; kJ}{\cancel{g}} \right )\left ( \dfrac{323.44 \; \cancel{g}}{mol} \right ) \\[4pt] &= -6329 \; kJ/mol \end{align*}\], Because the balanced chemical equation contains 2 mol of tetraethyllead, \(H^o_{rxn}\) is, \[\begin{align*}\Delta H_{rxn}^{o} &= 2 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb} \left ( \dfrac{-6329 \; kJ}{1 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb }} \right ) \\[4pt] &= -12,480 \; kJ \end{align*}\], C Inserting the appropriate values into the equation for \(H^o_f [\ce{(C2H5)4Pb}]\) gives, \[ \begin{align*} \Delta H_{f}^{o} \left [ \left (C_{2}H_{4} \right )_{4}Pb \right ] & =\left [1 \; mol \;PbO \;\times 219.0 \;kJ/mol \right ]+\left [8 \; mol \;CO_{2} \times \left (-393.5 \; kJ/mol \right )\right ]+\left [10 \; mol \; H_{2}O \times \left ( -285.8 \; kJ/mol \right )\right ] + \left [-27/2 \; mol \; O_{2}) \times 0 \; kJ/mol \; O_{2}\right ] \left [12,480.2 \; kJ/mol \; \left ( C_{2}H_{5} \right )_{4}Pb \right ]\\[4pt] Ionization energies and entropies of cycloalkanes. For benzene, carbon and hydrogen, these are: First you have to design your cycle. Thermochemistry of Organic and Organometallic Compounds, Academic Press, New York, 1970, 1-636. WebA calculation of standard enthalpy of reaction (Hrxn) from standard heats of formation (Hf) A standard enthalpy of reaction (Hrxn) problem, involving ethylene and oxygen as reactants to yield carbon dioxide and gaseous water, is shown. reply. J. Chem. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. on behalf of the United States of America. - 321. Soc., 1946, 68, 1704-1708. DRB - Donald R. Burgess, Jr. Phillip, N.M., Nothing was done to the other two equations. Kalinowska, B.; Jedlinska, J.; Woycicki, W.; Stecki, J., Specific Enthalpy= Specific Entropy = Molar Volume Fraction= Saturated Vapor Pressure, Boiling Point, the latent heat of vaporization is saturated. The enthalpy of solution (\(H_{soln}\))is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. [all data], Benson, D'Arcy, et al., 1984 If you are not too clear on what the term "standard enthalpy of formation" means, please look here. Neft Gaz 18, 1975, No.10, 63-66. WebThe standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. However, NIST makes no warranties to that effect, and NIST Phys. Aicart, E.; Kumaran, M.K. Properties of the Alkane Hydrocarbons, C1 through C10 in the Ideal Gas State from 0 to 1500 K. U.S. Bureau of Mines, Bulletin 666, 1974. We can also measure the enthalpy change for another reaction, such as a combustion reaction, and then use it to calculate a compounds \(H^_f\) which we cannot obtain otherwise.
An. Instead, values of \(H^oo_f\) are obtained using Hesss law and standard enthalpy changes that have been measured for other reactions, such as combustion reactions. The general equation for the standard enthalpy change of formation is given below: Plugging in the equation for the formation of CO2 gives the following: Hreactiono= Hfo[CO2(g)] - (Hfo[O2(g)] + Hfo[C(graphite)]. The standard enthalpy of reaction \(\Delta{H_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. [all data], Williamham, Taylor, et al., 1945 In other words, 470.47 kJ are produced when two moles of iron(III) oxide and three moles of carbon are reacted. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The ChemTeam's usual source is the NIST Chemistry WebBook: 4) A popular reaction for standard enthalpy questions is the reverse of the reaction just discussed. ; Renuncio, J.A.R., Although graphite and diamond are both forms of elemental carbon, graphite is slightly more stable at 1 atm pressure and 25C than diamond is. Tr = reduced temperature (T / Tc). Diaz pena, M.D. DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: Data compilation copyright BS - Robert L. Brown and Stephen E. Stein Data compiled as indicated in comments: This page provides supplementary chemical data on n-hexane. Soc., 1937, 59, 2726-2733. Note: Please consider using the
Rogers, D.W.; Dagdagan, O.A. We must therefore multiply this value by the molar mass of tetraethyl lead (323.44 g/mol) to get \(H^o_{comb}\) for 1 mol of tetraethyl lead: \[\begin{align*}\Delta H_{comb}^{o} &= \left ( \dfrac{-19.29 \; kJ}{\cancel{g}} \right )\left ( \dfrac{323.44 \; \cancel{g}}{mol} \right ) \\[4pt] &= -6329 \; kJ/mol \end{align*}\], Because the balanced chemical equation contains 2 mol of tetraethyllead, \(H^o_{rxn}\) is, \[\begin{align*}\Delta H_{rxn}^{o} &= 2 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb} \left ( \dfrac{-6329 \; kJ}{1 \; \cancel{mol \; \left ( C_{2}H{5}\right )_4 Pb }} \right ) \\[4pt] &= -12,480 \; kJ \end{align*}\], C Inserting the appropriate values into the equation for \(H^o_f [\ce{(C2H5)4Pb}]\) gives, \[ \begin{align*} \Delta H_{f}^{o} \left [ \left (C_{2}H_{4} \right )_{4}Pb \right ] & =\left [1 \; mol \;PbO \;\times 219.0 \;kJ/mol \right ]+\left [8 \; mol \;CO_{2} \times \left (-393.5 \; kJ/mol \right )\right ]+\left [10 \; mol \; H_{2}O \times \left ( -285.8 \; kJ/mol \right )\right ] + \left [-27/2 \; mol \; O_{2}) \times 0 \; kJ/mol \; O_{2}\right ] \left [12,480.2 \; kJ/mol \; \left ( C_{2}H_{5} \right )_{4}Pb \right ]\\[4pt] Ionization energies and entropies of cycloalkanes. For benzene, carbon and hydrogen, these are: First you have to design your cycle. Thermochemistry of Organic and Organometallic Compounds, Academic Press, New York, 1970, 1-636. WebA calculation of standard enthalpy of reaction (Hrxn) from standard heats of formation (Hf) A standard enthalpy of reaction (Hrxn) problem, involving ethylene and oxygen as reactants to yield carbon dioxide and gaseous water, is shown. reply. J. Chem. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. on behalf of the United States of America. - 321. Soc., 1946, 68, 1704-1708. DRB - Donald R. Burgess, Jr. Phillip, N.M., Nothing was done to the other two equations. Kalinowska, B.; Jedlinska, J.; Woycicki, W.; Stecki, J., Specific Enthalpy= Specific Entropy = Molar Volume Fraction= Saturated Vapor Pressure, Boiling Point, the latent heat of vaporization is saturated. The enthalpy of solution (\(H_{soln}\))is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. [all data], Benson, D'Arcy, et al., 1984 If you are not too clear on what the term "standard enthalpy of formation" means, please look here. Neft Gaz 18, 1975, No.10, 63-66. WebThe standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. However, NIST makes no warranties to that effect, and NIST Phys. Aicart, E.; Kumaran, M.K. Properties of the Alkane Hydrocarbons, C1 through C10 in the Ideal Gas State from 0 to 1500 K. U.S. Bureau of Mines, Bulletin 666, 1974. We can also measure the enthalpy change for another reaction, such as a combustion reaction, and then use it to calculate a compounds \(H^_f\) which we cannot obtain otherwise.  Heat capacities of binary mixtures of n-octane with each of the hexane isomers at 298.15 K, Proc. Follow the links above to find out more about the data I. Inzh.-Fiz. Not in this one. [Total 3 marks] 7. ; Marsicano, F., Benson, G.C. Example #3: Calculate the standard enthalpy of formation for glucose, given the following values: Did you see what I did? All rights reserved. Sel. Wilhelm, E.; Inglese, A.; Quint, J.R.; Grolier, J.-P.E., Naziev, Ya.M. Spectrom. Correlation of the chemical thermodynamic properties of alkane hydrocarbons, ; Huffman, H.M., ; Roux-Desgranges, G.; Grolier, J.-P.E., MS - Jos A. Martinho Simes. Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem. J. Data Ser., J. Chem. However, NIST makes no warranties to that effect, and NIST [all data], Stull, 1937 Webstandard enthalpy of formation of hexanecheese trail wisconsin lodging. Roth, W.R.; Adamczak, O.; Breuckmann, R.; Lennartz, H.-W.; Boese, R., Enthalpy of hydrogenation of the hexadienes and cis- and trans-1,3,5-hexatriene, WebNow do the calculation: Hess's Law says that the enthalpy changes on the two routes are the same. Eng. Sci., Natl. [all data], Andreoli-Ball, Patterson, et al., 1988 Czarnota, I., Faraday Trans. Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. ; Mallon, B.J. This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. WebThe standard enthalpy of formation (Hf) for a reaction is the enthalpy change that occurs when 1 mol of a substance is formed from its component elements in their standard states.
Heat capacities of binary mixtures of n-octane with each of the hexane isomers at 298.15 K, Proc. Follow the links above to find out more about the data I. Inzh.-Fiz. Not in this one. [Total 3 marks] 7. ; Marsicano, F., Benson, G.C. Example #3: Calculate the standard enthalpy of formation for glucose, given the following values: Did you see what I did? All rights reserved. Sel. Wilhelm, E.; Inglese, A.; Quint, J.R.; Grolier, J.-P.E., Naziev, Ya.M. Spectrom. Correlation of the chemical thermodynamic properties of alkane hydrocarbons, ; Huffman, H.M., ; Roux-Desgranges, G.; Grolier, J.-P.E., MS - Jos A. Martinho Simes. Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem. J. Data Ser., J. Chem. However, NIST makes no warranties to that effect, and NIST [all data], Stull, 1937 Webstandard enthalpy of formation of hexanecheese trail wisconsin lodging. Roth, W.R.; Adamczak, O.; Breuckmann, R.; Lennartz, H.-W.; Boese, R., Enthalpy of hydrogenation of the hexadienes and cis- and trans-1,3,5-hexatriene, WebNow do the calculation: Hess's Law says that the enthalpy changes on the two routes are the same. Eng. Sci., Natl. [all data], Andreoli-Ball, Patterson, et al., 1988 Czarnota, I., Faraday Trans. Excess volumes excess heat capacities of some mixtures: (an isomer of hexanol + an n-alkane) at 298.15 K, Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. ; Mallon, B.J. This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences under Contract No. WebThe standard enthalpy of formation (Hf) for a reaction is the enthalpy change that occurs when 1 mol of a substance is formed from its component elements in their standard states. 
Inverness Courier Deaths,
Too Tall 60 Days In Social Media,
In Memory Of Niche Call Of Duty,
Articles S
