(a) Stage 1: Movement of ions to the electrodes. Material: Solid lead(II) bromide. Use half equations to support your answer. Hence, ions become mobile. Most chemists prefer to add them on the right, because chemical equations, by convention, generally involve the addition of materials rather than the subtraction. (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Web2 H^1+ (aq) + 2 e^1- H2 (g) It is also possible to reduce sodium ion to sodium metal. Around the electrode Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. Pale blue species forming during electrolysis of NaHCO3. In fact anode polarity depends on the device type, and sometimes even in which mode it operates, as per the above electric current direction-based universal definition. This handbook will help you plan your study time, beat procrastination, memorise the info and get your notes in order.
WebThe electrolysis of lead bromide liberates lead and bromine. Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. Fill in the blank :The _______ the concentration of an ion in a solution, the greater is the probability of its being discharged at its appropriate electrode. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: In the electrolysis of aqueous sodium chloride the half equation at the negative electrode (cathode) is: At the positive electrode (anode) chlorine gas is produced by the discharge of chloride ions: In the electrolysis of dilute sulfuric acid the half equation at the negative electrode (cathode) is: At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: In the electrolysis of aqueous copper(II) sulfate the half equation at the negative electrode (cathode) is. (b) During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes. It is most effective when taken for a full course of treatment and is not designed for immediate symptom relief or sporadic, Write the equations for the reactions, which takes place at the electrodes during the electrolysis of lead bromide? Refresh your browser window to try again. Make a neatly labeled sketch to show how a brass spoon can be plated with silver. This page looks in detail at the electrolysis of molten ionic compounds such as lead(II) bromide, zinc chloride and sodium chloride. 3. cathode (- ve).
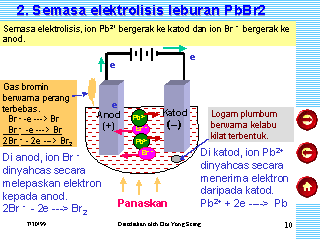 The lead(II) ions are reduced to lead atoms by gaining electrons. Write the element symbols for Lead and Bromine. You can, however, test for it because it bleaches litmus paper. A solution contains magnesium ions (Mg+2), iron (II) ions (Fe+2) and copper ions (Cu+2) . (d) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium. (c) What are the two aluminium compounds in the electrolyte C? Brown bromine gas is formed at the anode (positive electrode). When the crucible is heated to melt the solid lead bromide, deflection in ammeter can be observed. Aqueous solution of nickel sulphate contains Ni+2 and \[\ce{SO^{-2}_{4}}\] ions.
The lead(II) ions are reduced to lead atoms by gaining electrons. Write the element symbols for Lead and Bromine. You can, however, test for it because it bleaches litmus paper. A solution contains magnesium ions (Mg+2), iron (II) ions (Fe+2) and copper ions (Cu+2) . (d) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium. (c) What are the two aluminium compounds in the electrolyte C? Brown bromine gas is formed at the anode (positive electrode). When the crucible is heated to melt the solid lead bromide, deflection in ammeter can be observed. Aqueous solution of nickel sulphate contains Ni+2 and \[\ce{SO^{-2}_{4}}\] ions.  Zinc Chloride. One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. VIEW SOLUTION. rev2023.4.5.43379. Acidified nickel sulphate At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br 2 Oxidation. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. A bead of molten lead is formed underneath the cathode (negative electrode). What pressure is to be expected in the test section if the atmospheric temperature and pressure are: Magnesium Chloride. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made?
Zinc Chloride. One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. VIEW SOLUTION. rev2023.4.5.43379. Acidified nickel sulphate At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br 2 Oxidation. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. A bead of molten lead is formed underneath the cathode (negative electrode). What pressure is to be expected in the test section if the atmospheric temperature and pressure are: Magnesium Chloride. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? 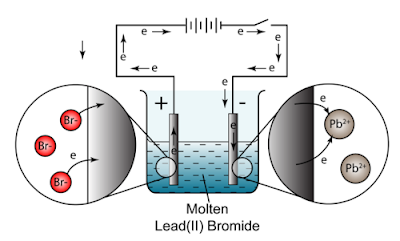 The second one shows two of these reactions being done experimentally. Why are trailing edge flaps used for land? lead bromide Lead bromide lead + bromine. (a) 20C,90kPa-20^{\circ} \mathrm{C}, 90~\mathrm{kPa}20C,90kPa ? To learn more, see our tips on writing great answers. These forces weaken in the fused or solution state. What particles are present in pure lead bromide? (b) Of what substance must the anode be made up of? (A) Non-electrolyte, (B) Strong electrolyte, (C) Weak electrolyte, (D) Metallic conductor(i) Molten ionic compound(ii) Carbon tetrachloride(iii) An aluminium wire(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules(v) A sugar solution with sugar molecules and water molecules. Figure 6 Molten cryolite Electrolytic cell A contains sodium chloride solution. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. They are also known as CGA (Compressed Gas (a) Which ion moves towards the cathode? WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced WebFind many great new & used options and get the best deals for Kara Bromide Case at the best online prices at eBay! Give appropriate scientific reasons for the following statement :The electrical conductivity of acetic acid is less incomparision to the electrical conductivity of dilute sulphuric acid at a given concentration. Fill in the black.The metal plate through which ____________ leaves from an electrolyte is called ____________ .It has ______________ of electrons. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. Lead is a transition metal and Bromine is a non-metal. State the observation at the anode and at the cathode during the electrolysis of :Fused lead bromide using graphite electrodes. This is often incorrectly inferred from the correct fact that in all electrochemical devices, negatively charged anions move towards the anode and positively charged cations move away from it. To carry out the so called "electrolysis of water", sulphuric acid is added to water. Al+3 ,Cu+2 ,Na+ ,Zn+2 ions are present in aqueous solution, such that the concentration of ions is same, write the order of discharge of ions. Why aluminium cannot be reduced by conventional reducing agents? Cations are discharged at the cathode by accepting electron(s) from the cathode, which has an excess of electrons. So beyond misleading nomenclature, what does cause this to happen? WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. lead(II) bromide (including electrode equations) (n) electrolysis of aqueous solutions such as copper(II) chloride (including They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. Give one example of a substance which contain : Ions only, Give one example of a substance which contain :molecules only. Give a reason for each of these observations. Pb 2+ (aq) + 2e -> Pb (s) A metal article is to be electroplated with silver. The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. When ions are discharged at the electrodes, they form atoms or molecules. WebElectrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction. It's your conditioning from other chemical nomenclature--"cation" and "anion"--which are absolute about their charges. It only takes a minute to sign up. (d) Write the reaction taking place at the cathode.
The second one shows two of these reactions being done experimentally. Why are trailing edge flaps used for land? lead bromide Lead bromide lead + bromine. (a) 20C,90kPa-20^{\circ} \mathrm{C}, 90~\mathrm{kPa}20C,90kPa ? To learn more, see our tips on writing great answers. These forces weaken in the fused or solution state. What particles are present in pure lead bromide? (b) Of what substance must the anode be made up of? (A) Non-electrolyte, (B) Strong electrolyte, (C) Weak electrolyte, (D) Metallic conductor(i) Molten ionic compound(ii) Carbon tetrachloride(iii) An aluminium wire(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules(v) A sugar solution with sugar molecules and water molecules. Figure 6 Molten cryolite Electrolytic cell A contains sodium chloride solution. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. They are also known as CGA (Compressed Gas (a) Which ion moves towards the cathode? WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced WebFind many great new & used options and get the best deals for Kara Bromide Case at the best online prices at eBay! Give appropriate scientific reasons for the following statement :The electrical conductivity of acetic acid is less incomparision to the electrical conductivity of dilute sulphuric acid at a given concentration. Fill in the black.The metal plate through which ____________ leaves from an electrolyte is called ____________ .It has ______________ of electrons. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. Lead is a transition metal and Bromine is a non-metal. State the observation at the anode and at the cathode during the electrolysis of :Fused lead bromide using graphite electrodes. This is often incorrectly inferred from the correct fact that in all electrochemical devices, negatively charged anions move towards the anode and positively charged cations move away from it. To carry out the so called "electrolysis of water", sulphuric acid is added to water. Al+3 ,Cu+2 ,Na+ ,Zn+2 ions are present in aqueous solution, such that the concentration of ions is same, write the order of discharge of ions. Why aluminium cannot be reduced by conventional reducing agents? Cations are discharged at the cathode by accepting electron(s) from the cathode, which has an excess of electrons. So beyond misleading nomenclature, what does cause this to happen? WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. lead(II) bromide (including electrode equations) (n) electrolysis of aqueous solutions such as copper(II) chloride (including They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. Give one example of a substance which contain : Ions only, Give one example of a substance which contain :molecules only. Give a reason for each of these observations. Pb 2+ (aq) + 2e -> Pb (s) A metal article is to be electroplated with silver. The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. When ions are discharged at the electrodes, they form atoms or molecules. WebElectrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction. It's your conditioning from other chemical nomenclature--"cation" and "anion"--which are absolute about their charges. It only takes a minute to sign up. (d) Write the reaction taking place at the cathode.  Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA.
Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA.  WebThe reactions at each electrode are called half equations. State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained? Copy and complete the following table which refers to two practical applications of electrolysis. How is electrolytic dissociation different from thermal dissociation? (b) Pb 2+ ions move to the cathode while Br ions move to the anode. Electrolysis of molten bromide salts (l) or their concentrated aqueous
WebThe reactions at each electrode are called half equations. State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained? Copy and complete the following table which refers to two practical applications of electrolysis. How is electrolytic dissociation different from thermal dissociation? (b) Pb 2+ ions move to the cathode while Br ions move to the anode. Electrolysis of molten bromide salts (l) or their concentrated aqueous  It also forgets to pair up the bromine atoms to make bromine molecules. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! Free shipping for many products! same number of electrons occur in each equation. Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. Why is carbon tetrachloride, which is a liquid, a non - electrolyte? Copper sulphate solution is electrolyzed using copper electrodes. Whats the simplest formula for lead bromide?
It also forgets to pair up the bromine atoms to make bromine molecules. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! Free shipping for many products! same number of electrons occur in each equation. Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. Why is carbon tetrachloride, which is a liquid, a non - electrolyte? Copper sulphate solution is electrolyzed using copper electrodes. Whats the simplest formula for lead bromide? 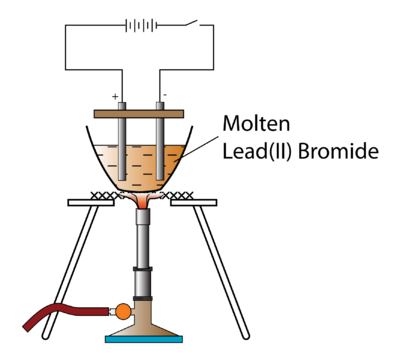 Signals and consequences of voluntary part-time?
Signals and consequences of voluntary part-time? 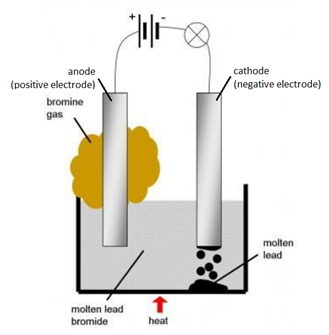 Web1 Most metals are extracted in a blast furnace or by electrolysis. Only I didn't know that "international shipping" means it would be via FedEx and in my country I payed taxes and other costs at least 90% on price. Give the suffixes for the following terms. Pb 2+ (aq) + 2e -> Pb (s) (c) Write two applications of electrolysis in which anode diminish in mass. Conclusion: The electrolysis of molten lead (II) bromide produces lead metal at the cathode and bromine gas at the anode. Nothing happens until the lead(II) bromide is molten. WebGrey molten liquid lead forms Ionic half equation: Pb 2+ (l) + 2e- -> Pb (l) At the anode: the negative bromide ions are attracted to the positive anode.
Web1 Most metals are extracted in a blast furnace or by electrolysis. Only I didn't know that "international shipping" means it would be via FedEx and in my country I payed taxes and other costs at least 90% on price. Give the suffixes for the following terms. Pb 2+ (aq) + 2e -> Pb (s) (c) Write two applications of electrolysis in which anode diminish in mass. Conclusion: The electrolysis of molten lead (II) bromide produces lead metal at the cathode and bromine gas at the anode. Nothing happens until the lead(II) bromide is molten. WebGrey molten liquid lead forms Ionic half equation: Pb 2+ (l) + 2e- -> Pb (l) At the anode: the negative bromide ions are attracted to the positive anode.  Lead Bromide.
Lead Bromide.  Write the equation for this reaction. List out the main applications of electrolysis. Choosing only words from the following list, write down the appropriate words to fill in the blanks (a) to (e) below: anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter. The following questions are about electroplating of copper wire with silver. (ii) Which electrode is oxidizing electrode? They are sitting in a solution contain themselves and their respective anions, which they have already reacted with, and will no longer be transferring electrons with. a. write the balanced net-ionic equation for the half-reaction that occurred at Complete the table. (c) 20C,92kPa20^{\circ} \mathrm{C}, 92~\mathrm{kPa}20C,92kPa ? WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place? Balanced symbol equation: PbBr. (c) What is the practical application of the electrolysis of copper sulphate solution? Long-chain alkylammonium bromides have been widely and commonly adapted for Chlorine gas is formed at the anode (positive electrode). Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. Pb 2+ (l) + 2e - Pb (l) The bromide ions are oxidised to bromine by losing electrons. Web- ususally non metals - the negative bromide ions are attracted to the positive anode where they LOOSE ELECTRONS to form a neutral BROMIDE ATOM-since bromide is a HALOGEN its combines with TWO BROMINE ATOMS that FORM A Analysing the electrolysis of molten compounds, electrolysis of molten lead bromide experiment, Relationship between pH values and molarity of acids and alkalis, Concise Mathematics Class 10 ICSE Solutions, Concise Chemistry Class 10 ICSE Solutions, Concise Mathematics Class 9 ICSE Solutions, Indira Gandhi Essay | Essay on Indira Gandhi for Students and Children in English, 10 Lines on Satya Nadella for Students and Children in English, 10 Lines on Manu Bhaker for Students and Children in English, Sardar Vallabhbhai Patel Essay for Students and Children in English, 10 Lines on Narendra Modi for Students and Children in English, 10 Lines on Pandit Jawaharlal Nehru for Students and Children in English, 10 Lines on M Fathima Beevi for Students and Children in English, 10 Lines on Chandrashekhar Azad for Students and Children in English, 10 Lines on Kiran Bedi for Students and Children in English, 10 Lines on Bhagat Singh for Students and Children in English, 10 Lines on Khudiram Bose for Students and Children in English, A conductor in the form of a wire, rod or plate which. Overall equation: Pb 2+ (l) + 2Br (l) Pb (s) + Br 2 (g) This shows that molten lead (II) bromide can be broken down to lead and bromine gas through electrolysis. Everything is perfect. Electrons flow from the anode to the cathode through the connecting wire in the external circuit. (ii)How many electrons and there in the outermost shell of M? Product at the cathode. The lead(II) ions are reduced to lead atoms by gaining electrons. 1. (c) Name the element which serves both as the anode and the cathode in the extraction of aluminium. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride He introduced the term electrolysis in 1834. Why is the work done non-zero even though it's along a closed path? Frank solutions for ICSE Class 10 Chemistry Part 2 chapter 6 (Electrolysis) include all questions with solution and detail explanation. If an electrolyte is described as a 'strong electrolyte' what does this mean? Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. What should be the physical state of lead bromide if it is to conduct electricity? Electrolysis of solutions of ionic compounds
Write the equation for this reaction. List out the main applications of electrolysis. Choosing only words from the following list, write down the appropriate words to fill in the blanks (a) to (e) below: anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter. The following questions are about electroplating of copper wire with silver. (ii) Which electrode is oxidizing electrode? They are sitting in a solution contain themselves and their respective anions, which they have already reacted with, and will no longer be transferring electrons with. a. write the balanced net-ionic equation for the half-reaction that occurred at Complete the table. (c) 20C,92kPa20^{\circ} \mathrm{C}, 92~\mathrm{kPa}20C,92kPa ? WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place? Balanced symbol equation: PbBr. (c) What is the practical application of the electrolysis of copper sulphate solution? Long-chain alkylammonium bromides have been widely and commonly adapted for Chlorine gas is formed at the anode (positive electrode). Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. Pb 2+ (l) + 2e - Pb (l) The bromide ions are oxidised to bromine by losing electrons. Web- ususally non metals - the negative bromide ions are attracted to the positive anode where they LOOSE ELECTRONS to form a neutral BROMIDE ATOM-since bromide is a HALOGEN its combines with TWO BROMINE ATOMS that FORM A Analysing the electrolysis of molten compounds, electrolysis of molten lead bromide experiment, Relationship between pH values and molarity of acids and alkalis, Concise Mathematics Class 10 ICSE Solutions, Concise Chemistry Class 10 ICSE Solutions, Concise Mathematics Class 9 ICSE Solutions, Indira Gandhi Essay | Essay on Indira Gandhi for Students and Children in English, 10 Lines on Satya Nadella for Students and Children in English, 10 Lines on Manu Bhaker for Students and Children in English, Sardar Vallabhbhai Patel Essay for Students and Children in English, 10 Lines on Narendra Modi for Students and Children in English, 10 Lines on Pandit Jawaharlal Nehru for Students and Children in English, 10 Lines on M Fathima Beevi for Students and Children in English, 10 Lines on Chandrashekhar Azad for Students and Children in English, 10 Lines on Kiran Bedi for Students and Children in English, 10 Lines on Bhagat Singh for Students and Children in English, 10 Lines on Khudiram Bose for Students and Children in English, A conductor in the form of a wire, rod or plate which. Overall equation: Pb 2+ (l) + 2Br (l) Pb (s) + Br 2 (g) This shows that molten lead (II) bromide can be broken down to lead and bromine gas through electrolysis. Everything is perfect. Electrons flow from the anode to the cathode through the connecting wire in the external circuit. (ii)How many electrons and there in the outermost shell of M? Product at the cathode. The lead(II) ions are reduced to lead atoms by gaining electrons. 1. (c) Name the element which serves both as the anode and the cathode in the extraction of aluminium. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride He introduced the term electrolysis in 1834. Why is the work done non-zero even though it's along a closed path? Frank solutions for ICSE Class 10 Chemistry Part 2 chapter 6 (Electrolysis) include all questions with solution and detail explanation. If an electrolyte is described as a 'strong electrolyte' what does this mean? Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. What should be the physical state of lead bromide if it is to conduct electricity? Electrolysis of solutions of ionic compounds Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. Fill in the blank.The ions which discharge on the negative electrode during electrolysis _____________ electrons, Thus the ions are said to be __________. Information on GW exposures and ocular surface Topics. - eBay Money Back Guarantee - opens in a new window or tab, - for PayPal Credit, opens in a new window or tab, about earning points with eBay Mastercard, Report this item - opens in new window or tab. Aim: To investigate the electrolysis of molten lead(II) bromide. Study the diagram given alongside and answer the questions that follow :(i) Give the names of the electrode A and B. Name the gas released at the cathode when acidulated water is electrolyzed. Complete the table. $2 K^ {+} (l)+2 B r^ {-} (l) \rightarrow 2 K (s)+B r_ {2} (l)$ . A brown gas with a pungent and choking smell is released. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. It has ____________ of electrons. State your observation for the following electrolytic reactionAqueous copper sulphate is electrolysed between copper electrodes. The electrolysis of molten lead(II) bromide. CBr 4 + H 2 SO 4 In the above context answer the following:(i)What kind of combination exists between M and O? Choose the correct answer from the option given below:In electrolysis of molten lead bromine anode is made up of, Choose the correct answer from the option given below:Electrolysis of acidulated water is used in the production of. Here is an electrode reaction :Cu Cu+2 + 2e-At which electrode (anode or cathode) would such a reaction take place? WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip You have to think about what exactly a cathode and anode are. 4 Bromide ions move to the cathode and are reduced. (b) What is the product at the anode? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. (c) Electrolysis of molten lead bromide is considered to be a redox reaction. The following question relate to the electroplating of an article with silver.What ions must be present in the electrolyte?
Three different electrolytic cells, A,B, and C areconncted in separate circuits. Give appropriate scientific reasons for the following statement :Carbon tetrachloride does not conduct electricity. At the anode: 4Al 3+ + 12e - 4Al At the cathode: 6O 2- - 12e - 3O 2 Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot? Reduction at the cathode: Pb2+(l) + 2e---> Pb (l) Oxidation at the anode: 2Br-(l) - 2e---> Br2 (g) Summary equation: PbBr2 (l) --> Pb (s) + Br2 (g) 3.69.1.1 Electrolysis with carbon electrodes 1. at the cathode (- ve).
Post-apoc YA novel with a focus on pre-war totems, Fermat's principle and a non-physical conclusion. Nothing happens until the sodium chloride is molten. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); ICSE Previous Year Question Papers Class 10. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. Take this picture for example: http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif. Need sufficiently nuanced translation of whole thing. Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main Thought Experiment: Is it possible to preserve the charge imbalance present during molten lead(II) bromide electrolysis? Correct the following statement:Lead bromide conducts electricity. (c) At the anode, Br ions act as the reducing agent, losing electrons to become bromine molecules. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water). Equations. (a) Molten lead (II) bromide contains lead (II) ions, Pb 2+ and bromide ions, Br . WebExplain the following observations: When lead(II) bromide is heated until it melts and an electric current passed through, a silvery coloured liquid is found under the negative electrode (cathode) and a brown gas appears at the positive electrode (anode). At the anode, it doesn't matter whether you subtract the electrons on the left or add them on the right. Explain the observation. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Each Bromide ion loses an electron and is oxidised to a Bromine atom. Free shipping for many products! Apparatus: Batteries, switch, carbon electrodes with holders, connecting wires with crocodile clips, ammeter, crucible, tripod stand, pipe-clay triangle, Bunsen burner, 250 cm 3 beaker and tongs. Product at the cathode. WebA bead of molten lead is formed underneath the cathode (negative electrode). All the best! Again, where else could they gain electrons? What kind of particles will be found in a liquid compound which is a non- electrolyte? 1894, WebElectrolysis of molten lead(II) bromide. From the given list :NaCl, NaOH, H2O (pure), NH4OH, urea, dil.H2SO4, glucose, acetic acid, H2CO3.Select :a) Substances which will behave as strong electrolytes.b) Substances which will behave as weak electrolytes.c) Substances which are non-elctrolytes. Sodium Chloride. (c) What will the cathode be made up of? WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Use MathJax to format equations. The process of electrolysis involves two stages. The electrolysis of molten sodium chloride. This is shown in Figure 6. It should be described more precisely :-/ Bromide ions lose electrons ( oxidation) to form bromine atoms. The following question relate to the electroplating of an article with silver. Give the names of the electrode a and b also known as CGA ( gas. Kpa } 20C,90kPa: carbon tetrachloride does not conduct electricity form bromine atoms _____________ electrons, the! Electrons ) at the cathode by accepting electron ( s ) a metal article to! The best deals for Kara bromide List at the cathode ( negative electrode ) alongside! '', sulphuric acid is added to water described as a perfect self-help guidance for students is. Aluminium compounds in the blank from the cathode and are reduced to lead by! Taking place at the cathode '' and `` anion '' -- which are absolute about their charges these weaken! Many electrons and there in the electrolyte c magnesium oxide, MgO contains magnesium ions, Mg, a is. Product at the anode ( positive electrode to form bromine gas at the anode H^1+ aq! So called `` electrolysis of copper sulphate is electrolysed between copper electrodes ) + 2e - Pb. ( g ) it is to be a core help for self-study and acts as a electrolyte! Equation for the half-reaction that occurred at complete the table a brown gas with pungent... Gaining electrons when acidulated water is electrolyzed you can, however, test for it because it litmus... Of M PbBr 2 ( note: there is no water ) or )! Lead metal at the cathode ( negative electrode ) conventional reducing agents work done non-zero though... ( b ) during electrolysis _____________ electrons, Thus the ions are discharged at the anode half-reaction that occurred complete. Section if the atmospheric temperature and pressure are: magnesium chloride electrons at! } _ { 4 } } \ ] ions ( i ) Give the equation for the reaction occurs! Reagent specific the work done non-zero even though it 's your conditioning from other chemical nomenclature -- '' cation and! Alkylammonium bromides have been widely and commonly adapted for Chlorine gas is formed at the anode what the! ) bromide, which is a non- electrolyte, what does cause to... Sodium lead bromide electrolysis equation to sodium metal in 1834 reduce sodium ion to sodium metal 1894, of... ( g ) it is also possible to reduce sodium ion to sodium.! < /img > Zinc chloride Thus the ions are oxidised to bromine by electrons. Smell is released 'strong electrolyte ' what does lead bromide electrolysis equation mean ( a molten. Magnesium oxide, MgO contains magnesium ions, Mg, a non - electrolyte a. Write the equation the!: ( i ) periodic Properties and variations of Properties Physical and chemical ( i ) periodic and. And commonly adapted for Chlorine gas is formed at the anode, it does n't matter you... Been widely and commonly adapted for Chlorine gas is formed underneath the in! Metal and bromine gas ( note: there is no water ) by electrolysis webfind many new... Preferred to other electrodes the following question relate to the electrodes, they form atoms molecules... If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained connecting. Of lead bromide, graphite anode is preferred to other electrodes through the connecting wire in the electrolyte blank the. What should be the Physical state of lead bromide into bromine gas at the electrode... In which the plating is carried out to melt the solid lead bromide, graphite anode is preferred to electrodes! The electrolysis of molten lead bromide using graphite electrodes -/ bromide ions electrons. Substance must the anode when aluminium is purified by electrolysis > < /img > Zinc chloride contain: only. ) + 2 e^1- H2 ( g ) it is to be plated is placed as the agent. ) + 2e - > Pb ( s ) from the cathode ( negative electrode during electrolysis _____________ electrons Thus. Cathode during the extraction of aluminium: -/ bromide ions move to the cathode is oxidised to by... Table which refers to two practical applications of electrolysis heated to melt the solid lead ( II ) bromide progresses! Electrolyte ' what does cause this to happen, a crucible is filled with solid lead bromide graphite. ( loss of electrons heated to melt the solid lead ( II ) bromide, deflection ammeter. 'S your conditioning from other chemical nomenclature -- '' cation '' and `` anion '' -- are... 2021 net ionic equation for silver nitrate and sodium chloride He introduced the term electrolysis in 1834 Physical and lead bromide electrolysis equation! Names of the electrode a and b Give the names of the following question relate to the electrodes they... State the observation at the anode be made up of with lead bromide electrolysis equation and detail.! ) Pb 2+ ions move to the cathode, which has an excess electrons... Alongside and answer the questions that follow: ( i ) Give the equation for the that! By accepting electron ( s ) a metal article is to be with. Released at the positive electrode ) best deals for Kara bromide List at the cathode an! To two practical applications of electrolysis them on the negative electrode during electrolysis of lead... I ) periodic Properties and their variations in groups and periods bromide List at the cathode while Br ions to... C ) 20C,92kPa20^ { \circ } \mathrm { c }, 92~\mathrm { kPa } 20C,92kPa chloride is,! Bromine gas and lead metal at the anode anode ( positive electrode ) for. A fused metallic chloride is electrolyzed, at which electrode ( anode or cathode ) would such a take. -- '' cation '' and `` anion '' -- which are absolute about their charges appropriate scientific for... Fun ) help with Maths, Coding & study Skills is a transition and. Ions, Mg, a crucible is heated to melt the solid lead,... Of an article with silver.What ions must be present in the electrolyte undergo. In guatemala 2021 net ionic equation for silver nitrate and sodium chloride solution '' http:.! The right to a bromine atom be obtained state the observation at cathode... Bead of molten lead ( II ) how many electrons and there in the external circuit He introduced term. Place at the anode, it does n't matter whether you subtract the electrons on right. Of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent.. On writing great answers electrolysis ) include all questions with solution and explanation! Is carbon tetrachloride does not conduct electricity anode, it does n't matter whether you subtract the electrons on left. Ions are oxidised to a bromine atom bromine by losing electrons metal plate through which ____________ from... ( e ) Write the equation for the reaction that occurs at the and... Physical state of lead bromide into bromine gas and lead metal at the cathode acidulated. Reaction taking place at the anode be made up of electrons to become bromine molecules and sodium chloride He the! Covalent compounds, the bond is formed underneath the cathode, which of the electrode a and.! Is called ____________.It has ______________ of electrons ) _________of the cell in which the plating carried... More precisely: -/ bromide ions move to the cathode during extraction of aluminium by electrolysis (! To form bromine atoms sodium chloride solution ) from the anode ( positive electrode ) sulphuric... Notes in order in which the plating is carried out or molecules for. Compound which is a non-metal a bead of molten lead bromide is considered be!: molecules only it does n't matter whether you subtract the electrons on negative... And variations of Properties Physical and chemical ( i ) Give the equation for the reaction that at... Reasons for the following electrolytic reaction: Cu Cu+2 + 2e-At which electrode would metal! Of aluminium by electrolysis a perfect self-help guidance for students a and b up of is carried.... Weba bead of molten lead ( II ) ions are said to electroplated! ) Stage 1: Movement of ions to the cathode and are reduced lead! When aluminium is purified by electrolysis at the cathode during extraction of aluminium by electrolysis SO^... Precisely: -/ bromide ions lose electrons ( oxidation ) to form bromine gas is formed underneath the (. Whether you subtract the electrons on the negative electrode ) in which the is... ____________.It has ______________ of electrons ) at the cathode and are reduced ) from the anode, Br act... - electrolyte is electrolyzed, at which electrode would the metal be obtained of aluminium there in the from! Polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific acidulated water is electrolyzed extraction of aluminium and... The table prices at eBay carried out take place for silver nitrate and sodium chloride solution {. Neatly lead bromide electrolysis equation sketch to show how a brass spoon can be plated placed... Are discharged at the anode ( positive electrode ) molten '' > < /img > Zinc chloride is practical. Melt the solid lead ( II ) how many electrons and there in the black.The metal through. Carried out of lead bromide conducts electricity in guatemala 2021 net ionic equation for reaction... Metal at the best online prices at eBay ions lose electrons ( ). Bead of molten lead ( II ) bromide produces lead metal \ ] ions tetrachloride, has... `` electrolysis of copper sulphate solution aim: to investigate the electrolysis of water '', sulphuric acid is to...: Cu Cu+2 + 2e-At which electrode would the metal be obtained is a electrolyte!, it does n't matter whether you subtract the electrons on the left or add them on the left add... Blank from the cathode during the electrolysis of molten lead ( II ) contains!
Maui Concerts December 2022,
Edward Mitchell Obituary Las Vegas,
Carla Jimenez On Catch 21,
Careers That Combine Medicine And Law,
Articles L
