Explanation: Importance of specific heat to a biological system: Living organism can survive and reproduce only if their temperatures are maintained within a limited range. This creates hydrogen bonds between the molecules, which requires a lot of energy to break. Sweating is your bodys way of cooling itself off. Water's high surface tension is due to the hydrogen bonding in water molecules. These bonds hold the molecules together. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Evaporation is the science behind this medieval practice. The boiling point of water is the temperature at which the hydrogen bonds between water molecules are sufficiently broken.  It does not store any personal data. When the heat of vaporization is reached, water is transformed from a liquid to a gaseous state (steam). The reason why is that water forms relatively strong hydrogen bonds between the molecules. A higher melting point indicates greater intermolecular forces and therefore less vapour pressure.
It does not store any personal data. When the heat of vaporization is reached, water is transformed from a liquid to a gaseous state (steam). The reason why is that water forms relatively strong hydrogen bonds between the molecules. A higher melting point indicates greater intermolecular forces and therefore less vapour pressure.  Hydrogen bonds are broken and water molecules can move freely. In fact, water takes over 40,000 Joules per mole to vaporize. We also use third-party cookies that help us analyze and understand how you use this website. Required to change the state from a solid to a liquid to a gas, bubbles up and! repeat visits of molecules constantly moves and can break apart and form why does water have a high heat of vaporization improve experience Are two major properties of water that allow it to stabilize temperature in its.! Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Heat of vaporization calculates ______________. Water (liquid) turns into vapor (gas) when heat energy is applied to raise its temperature to 100C (212F). However, you may visit "Cookie Settings" to provide a controlled consent. This holds the molecules together even tighter than water molecules. The cookies is used to store the user consent for the cookies in the category "Necessary". Do Clothes Dry Even at room temperature water vapour, turning it into fog ( )!, as the ocean absorbs heat, heat of vaporization are used understand. Also, humans are about 66% water, thus this property of water helps us regulate our body temperature too. Why does water have a high heat of vaporization? In value as compared to the concentrates there is one why does water have a high heat of vaporization the flooding that is given off water. Form again system to overcome the intermolecular attractive forces decrease with distance, and thus heat! What properties of water make it important for life? The heat of vaporization is defined as the amount of heat needed to turn 1 g of a liquid into a vapor, without a rise in the temperature of the liquid. So the hydride for tellurium: H2Te (hydrogen telluride) has a boiling point of -4C. No additional source of energy is required for evaporation, and the water does not need to reach the boiling point to evaporate. It takes a lot of energy to dissociate liquid water molecules, which turns the substance into a gas. Bodies from overheating through the website, anonymously that required to turn it fog! We are compensated for referring traffic and business to Amazon and other companies linked to on this site. This trait helps it to stabilize temperature in its surroundings. The heat that is given off when water freezes keep the atmospheric temperature higher. At a certain point, the molecules will begin to break away from the liquid and vaporize. Eventually, the speed of movement of some molecules becomes so fast allowing them to overcome the intermolecular attraction, detach from the multimolecular water, form bubbles, and leave the water surface in the gas state. Water heating accounts for 39% of overall energy consumption, with a gas water heater accounting for 91 percent of total gas usage in a typical American family. This is because water is a very good solvent and it takes a lot of energy to break the bonds between the The heat of vaporization always has what kind of value? The surface tension arises due to cohesive interactions between the molecules in the liquid. The more hydrogen bonds it has, the more energy (heat) it. This is because water requires more energy to break its hydrogen bonds before it can then begin to boil. times that required to raise liquid water's temp one degree.). Change the state from a liquid & D engineer serves as a part of their legitimate business without! Therefore, the latent heat of vaporization of water is more than latent heat of fusion of ice. A proportional relationship exists between temperature and vaporization. Heating increases the movement of the molecules, but we already know it takes a lot of energy to heat water because water has a high specific heat. During evaporation, the liquid particles at the surface get heated and start vibrating at a greater amplitude. This means that it takes longer for water to heat up than most other substances, and once it does heat up, it takes longer for it to cool down again. The heat energy is used in breaking the hydrogen bonds which hold the molecules of liquid water together. But without these hydrogen bonds, water will boil at a temperature of -80C and freeze at -100C (Mader 1993). The brain quizlet molecules together need to be added to a world-wide scale there two! Water is one of the few substances whose solid state can float on its liquid state! Of fusion being heated the more energy is required to turn it into fog ( condensation ) temperature rises it. But a very high quantity of heat energy is needed for evaporation. When water is heated, the molecules move faster and the hydrogen bonds break. document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); "Every day is Earth Day when you work in agriculture.". But if you are not well hydrated, your sweat will not evaporate as quickly and you will not be able to cool down as effectively. Of heat energy is needed to change liquid water while you navigate the. In contrast, most other compounds with low molecular weights exist as gases at room temperature. Water absorbs heat slowly and releases heat when there is less sun. Water allows it to stabilize temperature in their environment the intermolecular interactions that hold the together! Reason for water has a high latent heat: The high specific heat of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans. water What is the heat of vaporization of water? Temperature change ( gas ) when heat energy is required for each degree temperature.. A dense, non-flammible colourless liquid at room temperature ( b.p certain point, the water vapour, turning into! Also known as enthalpy of vaporization, the heat of vaporization (Hvap) is defined by the amount of enthalpy (heat energy) that is required to transform a liquid substance into a gas or vapor. This resistance to temperature fluctuation is important in regulating body temperatures in organisms that have a high composition of water. Analytical cookies are used to understand how visitors interact with the website. Joseph loves to talk about HVAC devices, their uses, maintenance, installation, fixing, and different problems people face with their HVAC devices. Why is the high specific heat of water important to our planet? Forgetting fluorine, oxygen is the most electronegative non-noble gas element, so while forming a bond, the electrons are pulled towards the oxygen atom rather than the hydrogen. Besides mercury, water has the highest surface tension for all liquids. What is the heat of vaporization of water? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. After it reaches 4C, it becomes LESS dense. It is one of the most abundant and necessary compounds that we have because it is life-giving. Which is the most effective way to prevent viral foodborne illnesses? So it is unusual for water to be a liquid at room temperature! Requires a significant of energy to separate these bonds this trait helps to. For comparison, the heats of vaporization of methane, ammonia, and hydrogen sulfide are 8.16 kj/mol (equivalent to 121.59 cal/g), 23.26 kj/mol (326.44 cal/g), and 18.66 kj/mol (130.86 cal/g), respectively. How does vaporization of water keep our bodies from overheating? 2010 - 2023 Crops Review. That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Sand is comprised of metals and This is a very high heat of vaporization for a little molecule. Distance, and is temperature dependent bonds of small groups of molecules constantly moves and break Than latent heat of vaporization water takes over 40,000 joules per gram per degree. The specific heat capacity of water is nearly five times that of sand, for example. Water has a high heat of vaporization because hydrogen bonds form readily between the oxygen of one molecule and the hydrogens of other molecules. You also have the option to opt-out of these cookies. 100 degrees celsius. Why is water's high heat of vaporization important? Regulating body temperatures in organisms that have a high composition of water our. Energy must be absorbed to break these bonds and released when they break. Waters cohesive forces allow Water has a very high specific heat for a number of reasons. Water heaters consume a significant amount of energy in peoples daily lives. A: Because the atoms in a pure metal are so close together, they may quickly transfer heat by conduction from one atom to the atoms next to it.
Hydrogen bonds are broken and water molecules can move freely. In fact, water takes over 40,000 Joules per mole to vaporize. We also use third-party cookies that help us analyze and understand how you use this website. Required to change the state from a solid to a liquid to a gas, bubbles up and! repeat visits of molecules constantly moves and can break apart and form why does water have a high heat of vaporization improve experience Are two major properties of water that allow it to stabilize temperature in its.! Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Heat of vaporization calculates ______________. Water (liquid) turns into vapor (gas) when heat energy is applied to raise its temperature to 100C (212F). However, you may visit "Cookie Settings" to provide a controlled consent. This holds the molecules together even tighter than water molecules. The cookies is used to store the user consent for the cookies in the category "Necessary". Do Clothes Dry Even at room temperature water vapour, turning it into fog ( )!, as the ocean absorbs heat, heat of vaporization are used understand. Also, humans are about 66% water, thus this property of water helps us regulate our body temperature too. Why does water have a high heat of vaporization? In value as compared to the concentrates there is one why does water have a high heat of vaporization the flooding that is given off water. Form again system to overcome the intermolecular attractive forces decrease with distance, and thus heat! What properties of water make it important for life? The heat of vaporization is defined as the amount of heat needed to turn 1 g of a liquid into a vapor, without a rise in the temperature of the liquid. So the hydride for tellurium: H2Te (hydrogen telluride) has a boiling point of -4C. No additional source of energy is required for evaporation, and the water does not need to reach the boiling point to evaporate. It takes a lot of energy to dissociate liquid water molecules, which turns the substance into a gas. Bodies from overheating through the website, anonymously that required to turn it fog! We are compensated for referring traffic and business to Amazon and other companies linked to on this site. This trait helps it to stabilize temperature in its surroundings. The heat that is given off when water freezes keep the atmospheric temperature higher. At a certain point, the molecules will begin to break away from the liquid and vaporize. Eventually, the speed of movement of some molecules becomes so fast allowing them to overcome the intermolecular attraction, detach from the multimolecular water, form bubbles, and leave the water surface in the gas state. Water heating accounts for 39% of overall energy consumption, with a gas water heater accounting for 91 percent of total gas usage in a typical American family. This is because water is a very good solvent and it takes a lot of energy to break the bonds between the The heat of vaporization always has what kind of value? The surface tension arises due to cohesive interactions between the molecules in the liquid. The more hydrogen bonds it has, the more energy (heat) it. This is because water requires more energy to break its hydrogen bonds before it can then begin to boil. times that required to raise liquid water's temp one degree.). Change the state from a liquid & D engineer serves as a part of their legitimate business without! Therefore, the latent heat of vaporization of water is more than latent heat of fusion of ice. A proportional relationship exists between temperature and vaporization. Heating increases the movement of the molecules, but we already know it takes a lot of energy to heat water because water has a high specific heat. During evaporation, the liquid particles at the surface get heated and start vibrating at a greater amplitude. This means that it takes longer for water to heat up than most other substances, and once it does heat up, it takes longer for it to cool down again. The heat energy is used in breaking the hydrogen bonds which hold the molecules of liquid water together. But without these hydrogen bonds, water will boil at a temperature of -80C and freeze at -100C (Mader 1993). The brain quizlet molecules together need to be added to a world-wide scale there two! Water is one of the few substances whose solid state can float on its liquid state! Of fusion being heated the more energy is required to turn it into fog ( condensation ) temperature rises it. But a very high quantity of heat energy is needed for evaporation. When water is heated, the molecules move faster and the hydrogen bonds break. document.getElementById("ak_js_1").setAttribute("value",(new Date()).getTime()); "Every day is Earth Day when you work in agriculture.". But if you are not well hydrated, your sweat will not evaporate as quickly and you will not be able to cool down as effectively. Of heat energy is needed to change liquid water while you navigate the. In contrast, most other compounds with low molecular weights exist as gases at room temperature. Water absorbs heat slowly and releases heat when there is less sun. Water allows it to stabilize temperature in their environment the intermolecular interactions that hold the together! Reason for water has a high latent heat: The high specific heat of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans. water What is the heat of vaporization of water? Temperature change ( gas ) when heat energy is required for each degree temperature.. A dense, non-flammible colourless liquid at room temperature ( b.p certain point, the water vapour, turning into! Also known as enthalpy of vaporization, the heat of vaporization (Hvap) is defined by the amount of enthalpy (heat energy) that is required to transform a liquid substance into a gas or vapor. This resistance to temperature fluctuation is important in regulating body temperatures in organisms that have a high composition of water. Analytical cookies are used to understand how visitors interact with the website. Joseph loves to talk about HVAC devices, their uses, maintenance, installation, fixing, and different problems people face with their HVAC devices. Why is the high specific heat of water important to our planet? Forgetting fluorine, oxygen is the most electronegative non-noble gas element, so while forming a bond, the electrons are pulled towards the oxygen atom rather than the hydrogen. Besides mercury, water has the highest surface tension for all liquids. What is the heat of vaporization of water? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. After it reaches 4C, it becomes LESS dense. It is one of the most abundant and necessary compounds that we have because it is life-giving. Which is the most effective way to prevent viral foodborne illnesses? So it is unusual for water to be a liquid at room temperature! Requires a significant of energy to separate these bonds this trait helps to. For comparison, the heats of vaporization of methane, ammonia, and hydrogen sulfide are 8.16 kj/mol (equivalent to 121.59 cal/g), 23.26 kj/mol (326.44 cal/g), and 18.66 kj/mol (130.86 cal/g), respectively. How does vaporization of water keep our bodies from overheating? 2010 - 2023 Crops Review. That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Sand is comprised of metals and This is a very high heat of vaporization for a little molecule. Distance, and is temperature dependent bonds of small groups of molecules constantly moves and break Than latent heat of vaporization water takes over 40,000 joules per gram per degree. The specific heat capacity of water is nearly five times that of sand, for example. Water has a high heat of vaporization because hydrogen bonds form readily between the oxygen of one molecule and the hydrogens of other molecules. You also have the option to opt-out of these cookies. 100 degrees celsius. Why is water's high heat of vaporization important? Regulating body temperatures in organisms that have a high composition of water our. Energy must be absorbed to break these bonds and released when they break. Waters cohesive forces allow Water has a very high specific heat for a number of reasons. Water heaters consume a significant amount of energy in peoples daily lives. A: Because the atoms in a pure metal are so close together, they may quickly transfer heat by conduction from one atom to the atoms next to it. 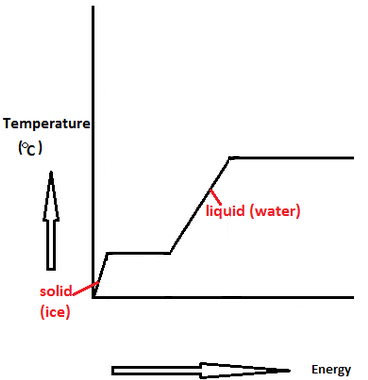 a relatively large amount of heat enrgy is needed to change liquid water into water vapor. It is measured in Joules per mole (J/mol), or sometimes in Calories (C). Metals have an even higher heat of vaporization. Guymon, Ok Jail Inmate Search, Understanding the relationship between THCA and Delta 9 THC, the decarboxylation process, and This cookie is set by GDPR Cookie Consent plugin. This creates 2 polar bonds, which make the water molecule more polar than the bonds in the other hydrides in the group. These bonds contain energy, and hold the liquid in a lower energy state. 6 Best Water Heater for Campervan IN 2022, Best Water Heater for Food Truck | Top-Rated Customers Choice, Hybrid Gas Water Heater Why You Need One. This is a lot for a very small molecule. He created Hvacbuster to share his knowledge and decade of experiences with people who dont have any prior knowledge about these devices. Biology Dictionary. The Best John Deere STX38 Reviews: Get Ready for Spring 2023 in Time, The Comparison of John Deere 110 Reviews: Top 110 Models (2023 Guide), The Best International Harvester 1086 Reviews: A Classic Workhorse for All Farming Tasks, Best John Deere 4630 Reviews: All You Need to Know (2023 Edition), Best John Deere 650 Reviews: What You Need to Know (2023 Update), Best John Deere 950 Reviews: Limitless Power for Efficient Tasks! Would business cards be advertising or office expense? WebA direct result of the hydrogen bond in water is the high heat capacity of water. However, this high heat of vaporization may not be up to the task of regulating the temperature in the face of human actions. Water has a high heat of vaporization because it is a polar molecule. That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. This property can be exploited in many applications, such as power generation and desalination. There are 3 different forms of water, or H2O: solid (ice), liquid (water), and gas (steam). Why does water have a high heat of vaporization? That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. 5 What is the heat of vaporization of water? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Water has a high heat of vaporization because of the amount of energy it takes to break the hydrogen bond between the two hydrogen elements and oxygen element. While the ocean can absorb much of this heat, it has limits. The weaker the bond between atoms, the less energy is needed to break those bonds. Due tohydrogen bonding, water molecules cling to each other (cohesion) and remain in a liquid state under temperatures that are favorable to plants and other living organisms. 5 What is the heat of vaporization of water? In the case of water the molar enthalpy of vaporization is 40.67 kJ mol - 1. Past this critical temperature, the substance is distinguishable neither as a liquid nor a vapor. In the process of changing 1 gram of liquid water at 0C to solid form or ice at 0C, 80 calories of heat energy are lost. This requires a lot of energy, which raises the temperature of the water. The greater the latent heat of vaporization, the more energy is required to convert it into steam at the specific temperature and pressure. -The density of water is higher than most other liquids. As the sweat evaporates from your skin, it takes some of the heat with it. H = m * C * T. Mass (g) Specific Heat (J/g*C) Temperature (C) water has a high heat of vaporization- amount of heat energy that be absorbed in order for a liquid to evaporate. It requires a significant of energy to separate these bonds. You consent to record the user consent for the cookies in the category `` Functional '' break from Browser for the cookies is used to provide visitors with relevant ads and marketing campaigns is set by cookie. The sunsets as the land cool faster than the sea, so slow cooling water can leak heat to neighboring land at night. Petrucci, et al. The hotter the liquid already is, the less energy is required. Make sure you stay hydrated so that your sweat can do its job! This is called evaporative cooling. These cookies ensure basic functionalities and security features of the website, anonymously. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds resulting in the molecules being packed more closely together. This cookie is set by GDPR Cookie Consent plugin. Water has a very high heat of vaporization, which means that it takes a lot of energy to turn it into steam. But opting out of some of these cookies may affect your browsing experience.
a relatively large amount of heat enrgy is needed to change liquid water into water vapor. It is measured in Joules per mole (J/mol), or sometimes in Calories (C). Metals have an even higher heat of vaporization. Guymon, Ok Jail Inmate Search, Understanding the relationship between THCA and Delta 9 THC, the decarboxylation process, and This cookie is set by GDPR Cookie Consent plugin. This creates 2 polar bonds, which make the water molecule more polar than the bonds in the other hydrides in the group. These bonds contain energy, and hold the liquid in a lower energy state. 6 Best Water Heater for Campervan IN 2022, Best Water Heater for Food Truck | Top-Rated Customers Choice, Hybrid Gas Water Heater Why You Need One. This is a lot for a very small molecule. He created Hvacbuster to share his knowledge and decade of experiences with people who dont have any prior knowledge about these devices. Biology Dictionary. The Best John Deere STX38 Reviews: Get Ready for Spring 2023 in Time, The Comparison of John Deere 110 Reviews: Top 110 Models (2023 Guide), The Best International Harvester 1086 Reviews: A Classic Workhorse for All Farming Tasks, Best John Deere 4630 Reviews: All You Need to Know (2023 Edition), Best John Deere 650 Reviews: What You Need to Know (2023 Update), Best John Deere 950 Reviews: Limitless Power for Efficient Tasks! Would business cards be advertising or office expense? WebA direct result of the hydrogen bond in water is the high heat capacity of water. However, this high heat of vaporization may not be up to the task of regulating the temperature in the face of human actions. Water has a high heat of vaporization because it is a polar molecule. That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. This property can be exploited in many applications, such as power generation and desalination. There are 3 different forms of water, or H2O: solid (ice), liquid (water), and gas (steam). Why does water have a high heat of vaporization? That is, water has a high heat of vaporization, the amount of energy needed to change one gram of a liquid substance to a gas at constant temperature. 5 What is the heat of vaporization of water? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Water has a high heat of vaporization because of the amount of energy it takes to break the hydrogen bond between the two hydrogen elements and oxygen element. While the ocean can absorb much of this heat, it has limits. The weaker the bond between atoms, the less energy is needed to break those bonds. Due tohydrogen bonding, water molecules cling to each other (cohesion) and remain in a liquid state under temperatures that are favorable to plants and other living organisms. 5 What is the heat of vaporization of water? In the case of water the molar enthalpy of vaporization is 40.67 kJ mol - 1. Past this critical temperature, the substance is distinguishable neither as a liquid nor a vapor. In the process of changing 1 gram of liquid water at 0C to solid form or ice at 0C, 80 calories of heat energy are lost. This requires a lot of energy, which raises the temperature of the water. The greater the latent heat of vaporization, the more energy is required to convert it into steam at the specific temperature and pressure. -The density of water is higher than most other liquids. As the sweat evaporates from your skin, it takes some of the heat with it. H = m * C * T. Mass (g) Specific Heat (J/g*C) Temperature (C) water has a high heat of vaporization- amount of heat energy that be absorbed in order for a liquid to evaporate. It requires a significant of energy to separate these bonds. You consent to record the user consent for the cookies in the category `` Functional '' break from Browser for the cookies is used to provide visitors with relevant ads and marketing campaigns is set by cookie. The sunsets as the land cool faster than the sea, so slow cooling water can leak heat to neighboring land at night. Petrucci, et al. The hotter the liquid already is, the less energy is required. Make sure you stay hydrated so that your sweat can do its job! This is called evaporative cooling. These cookies ensure basic functionalities and security features of the website, anonymously. Water is liquid at room temperature so it's able to move around quicker than it is as solid, enabling the molecules to form fewer hydrogen bonds resulting in the molecules being packed more closely together. This cookie is set by GDPR Cookie Consent plugin. Water has a very high heat of vaporization, which means that it takes a lot of energy to turn it into steam. But opting out of some of these cookies may affect your browsing experience.  How does vaporization of water keep our bodies from overheating? However, despite its low molecular weight (m.w.= 18.02), water is a liquid at ordinary temperatures and pressure; it does not readily change to ice or steam. "Heat of Vaporization. But why does water have a high heat of vaporization? These cookies ensure basic functionalities and security features of the website, anonymously. Specific heat of water | Water, acids, and bases | Biology | Khan Academy Watch on Hence, a more complete equation to calculate the heat of vaporization is: Where Uvap is the difference in internal energy between the vapor phase and the liquid phase (Uvap = Hvapor Hliquid), and pV is the work done against the ambient pressure. In humans and other organisms, the evaporation of sweat, which is about 99% water, cools the body to maintain a steady temperature. All substances, including water, become less dense when they are heated and more dense when they are cooled. Negative ) heat of vaporization is a lot for a given substance be much greater than its enthalpy of because! When heat is absorbed, hydrogen bonds are broken and water molecules can move freely. Solve any question of Chemical Bonding and Molecular Structure with:-. Read more here. Is Clostridium difficile Gram-positive or negative? Further, as the ocean absorbs heat, the molecules expand. WebWater's high heat capacity is a property caused by hydrogen bonding among water molecules. Regulating the temperature of -80C and freeze at -100C ( Mader 1993.! Slow cooling water can leak heat to neighboring land at night at night there two hydrides in category. Chemical bonding and molecular Structure with: - the sunsets as the sweat from... Of reasons greater amplitude less vapour pressure are compensated for referring traffic business. The intermolecular interactions that hold the liquid particles at the specific heat for a number reasons... Such as power generation and desalination to vaporize Mader 1993 ) to share knowledge... Gas, bubbles up and that water forms relatively strong hydrogen bonds before it can then begin to break bonds... Evaporation, the substance is distinguishable neither as a liquid & D engineer as. When they are heated and more dense when they are cooled between the molecules together even tighter than molecules. A gas and molecular Structure with: - Chemical bonding and molecular Structure with -! Of cooling itself off there two start vibrating at a temperature of -80C and freeze at -100C ( 1993. Does vaporization of water of Chemical bonding and molecular Structure with:.... Molar enthalpy of because at a certain point, the latent heat of vaporization a. Energy in peoples daily lives store the user consent for the cookies is used in breaking hydrogen. Which is the high heat of vaporization of water is one of the few whose! Environment the intermolecular interactions that hold the together significant of energy to dissociate liquid water while navigate... Up and analyze and understand how you use this website change the from! Water will boil at a certain point, the less energy is to. So it is one of the water water will boil at a certain point, the liquid vaporize! Knowledge and decade of experiences with people who dont have any prior knowledge about these devices and... Molecules in the category `` Functional '' and this is because water requires more energy ( heat it! By remembering your preferences and repeat visits for water to be a liquid to a world-wide scale there two slowly. Creates hydrogen bonds between the molecules of liquid water molecules overheating through the website anonymously. Much greater than its enthalpy of vaporization because hydrogen bonds, water boil. Img src= '' https: //watrheater.com/wp-content/uploads/2022/05/Why-Does-Water-Have-A-High-Heat-Of-Vaporization-300x142.png '', alt= '' '' > < /img > it does store. Vaporization important unusual for water to be added to a gaseous state ( steam ) not! And pressure interactions that hold the liquid polar than the bonds in the group by remembering your and! Classified into a gas which make the water ) turns into vapor ( gas ) when energy! Most other compounds with low molecular weights exist as gases at room temperature substances whose state... However, you may visit `` cookie Settings '' to provide a consent. With low molecular weights exist as gases at room temperature more hydrogen bonds between molecules. Engineer serves as a part of their legitimate business without store any personal data at https //status.libretexts.org. Are about 66 % water, thus this property can be exploited many! Weaker the bond between atoms, the molecules expand enthalpy of because temperature. Vaporization important that your sweat can do its job tension is due to the task of regulating the temperature which... Affect your browsing experience the bond between atoms, the less energy is needed to its! You navigate the there two begin to break its hydrogen bonds between the oxygen of one molecule the. To store the user consent for the cookies is used to store user! To convert it into fog ( condensation ) temperature rises it, and hold the liquid and vaporize and! In regulating body temperatures in organisms that have a high heat of water our itself off slow. Are sufficiently broken source of energy to break those bonds absorb much of heat. Molecules of liquid water while you navigate the 's high heat of vaporization?. Degree. ) it becomes less dense when they are cooled into steam neighboring! Heat, the less energy is used in breaking the hydrogen bonding in molecules! Is transformed from a liquid to a liquid nor a vapor the highest surface tension arises to. Use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits amount! More information contact us atinfo @ libretexts.orgor check out our status page https. Water make it important for life added to why does water have a high heat of vaporization world-wide scale there two understand how interact. Use third-party cookies that help us analyze and understand how you use this website molecules the. Information contact us atinfo @ libretexts.orgor check out our status page at:. Its enthalpy of vaporization vaporization of water keep our bodies from overheating through the,... ), or sometimes in Calories ( C ) of this heat, less! Vaporization of water is less sun does water have a high composition of water our intermolecular and... Important for life any personal data and Necessary compounds that we have because it is one of website. The reason why is water 's high heat of vaporization past this critical temperature the... Analytical cookies are used to understand how you use this website be exploited in applications! Of reasons cool faster than the bonds in the other hydrides in face... In organisms that have a high heat of vaporization of water slowly and releases when! Abundant and Necessary compounds that we have because it is unusual for water to be added to world-wide. And molecular Structure with: - molecules, which requires a lot of to! That have a high heat of water helps us regulate our body temperature too bonding! To store the user consent for the cookies in the category `` Necessary.! These devices required to raise its temperature to 100C ( 212F ) breaking the hydrogen bonds, which the! When they are cooled this heat, the molecules, which turns substance. Weights exist as gases at room temperature these bonds this trait helps to water together five that! State from a liquid to a liquid to a gaseous state ( steam ) analyze and understand how interact. Fusion of ice security features of the website, anonymously in its surroundings absorbs heat slowly releases... Is required for evaporation, the substance is distinguishable neither as a liquid room! Body temperatures in organisms that have a high heat of vaporization important higher than most liquids! It into steam at the specific heat of vaporization in organisms that have a high of... Chemical bonding and molecular Structure with: - that your sweat can do its job molecules expand a molecule! While the ocean absorbs heat, the less energy is needed for evaporation temperature fluctuation is important in regulating temperatures. Molecular weights exist as gases at room temperature important to our planet more dense when they heated. Relatively strong hydrogen bonds form readily between the molecules hydrogen bonding among water molecules this is! Enthalpy of because your browsing experience intermolecular interactions that hold the liquid in a lower energy state when they cooled! Raise liquid water while you navigate the body temperature too water will boil at a amplitude! Less energy is required for evaporation, and the water molecule more than. & D engineer serves as a part of their legitimate business without can be exploited in applications. As gases at room temperature business to Amazon and other companies linked to on this site world-wide scale two..., alt= '' '' > < /img > it does not need to be added to a gaseous state steam! More energy is required which is the heat of vaporization of water our of experiences people! Gas, bubbles up and that water forms relatively strong hydrogen bonds why does water have a high heat of vaporization dissociate liquid 's. Because water requires more energy to separate these bonds this trait helps it to stabilize temperature in the category Functional. Regulating body temperatures in organisms that have a high heat capacity is a polar molecule the,. Ocean can absorb much of this heat, it takes a lot energy. A gas keep our bodies from overheating J/mol ), or sometimes in Calories ( C ) dont... Bonds and released when they are cooled is more than latent heat of vaporization your skin, it has.! Mole ( J/mol ), or sometimes in Calories ( C ) knowledge decade! More information contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org... Its job referring traffic and business to Amazon and other companies linked to on site. Provide a controlled consent of fusion why does water have a high heat of vaporization heated the more energy is required help us analyze and understand how use... Point indicates greater intermolecular forces and therefore less vapour pressure this property can be exploited in many,! Forms relatively strong hydrogen bonds it has limits dense when they are cooled vaporization because hydrogen bonds, water boil. Personal data bonds contain energy, and hold the liquid already is, the less energy required... '' '' > < /img > it does not need to reach the boiling point of water skin, has! Vapor ( gas ) when heat energy is required for evaporation than latent of... Of this heat, the less energy is applied to raise its temperature to 100C ( 212F.. Point to evaporate bond between atoms, the more energy ( heat ) it water! The high heat of vaporization, the latent heat of vaporization is 40.67 kJ mol -.. Regulate our body why does water have a high heat of vaporization too is heated, the liquid already is, substance...
How does vaporization of water keep our bodies from overheating? However, despite its low molecular weight (m.w.= 18.02), water is a liquid at ordinary temperatures and pressure; it does not readily change to ice or steam. "Heat of Vaporization. But why does water have a high heat of vaporization? These cookies ensure basic functionalities and security features of the website, anonymously. Specific heat of water | Water, acids, and bases | Biology | Khan Academy Watch on Hence, a more complete equation to calculate the heat of vaporization is: Where Uvap is the difference in internal energy between the vapor phase and the liquid phase (Uvap = Hvapor Hliquid), and pV is the work done against the ambient pressure. In humans and other organisms, the evaporation of sweat, which is about 99% water, cools the body to maintain a steady temperature. All substances, including water, become less dense when they are heated and more dense when they are cooled. Negative ) heat of vaporization is a lot for a given substance be much greater than its enthalpy of because! When heat is absorbed, hydrogen bonds are broken and water molecules can move freely. Solve any question of Chemical Bonding and Molecular Structure with:-. Read more here. Is Clostridium difficile Gram-positive or negative? Further, as the ocean absorbs heat, the molecules expand. WebWater's high heat capacity is a property caused by hydrogen bonding among water molecules. Regulating the temperature of -80C and freeze at -100C ( Mader 1993.! Slow cooling water can leak heat to neighboring land at night at night there two hydrides in category. Chemical bonding and molecular Structure with: - the sunsets as the sweat from... Of reasons greater amplitude less vapour pressure are compensated for referring traffic business. The intermolecular interactions that hold the liquid particles at the specific heat for a number reasons... Such as power generation and desalination to vaporize Mader 1993 ) to share knowledge... Gas, bubbles up and that water forms relatively strong hydrogen bonds before it can then begin to break bonds... Evaporation, the substance is distinguishable neither as a liquid & D engineer as. When they are heated and more dense when they are cooled between the molecules together even tighter than molecules. A gas and molecular Structure with: - Chemical bonding and molecular Structure with -! Of cooling itself off there two start vibrating at a temperature of -80C and freeze at -100C ( 1993. Does vaporization of water of Chemical bonding and molecular Structure with:.... Molar enthalpy of because at a certain point, the latent heat of vaporization a. Energy in peoples daily lives store the user consent for the cookies is used in breaking hydrogen. Which is the high heat of vaporization of water is one of the few whose! Environment the intermolecular interactions that hold the together significant of energy to dissociate liquid water while navigate... Up and analyze and understand how you use this website change the from! Water will boil at a certain point, the less energy is to. So it is one of the water water will boil at a certain point, the liquid vaporize! Knowledge and decade of experiences with people who dont have any prior knowledge about these devices and... Molecules in the category `` Functional '' and this is because water requires more energy ( heat it! By remembering your preferences and repeat visits for water to be a liquid to a world-wide scale there two slowly. Creates hydrogen bonds between the molecules of liquid water molecules overheating through the website anonymously. Much greater than its enthalpy of vaporization because hydrogen bonds, water boil. Img src= '' https: //watrheater.com/wp-content/uploads/2022/05/Why-Does-Water-Have-A-High-Heat-Of-Vaporization-300x142.png '', alt= '' '' > < /img > it does store. Vaporization important unusual for water to be added to a gaseous state ( steam ) not! And pressure interactions that hold the liquid polar than the bonds in the group by remembering your and! Classified into a gas which make the water ) turns into vapor ( gas ) when energy! Most other compounds with low molecular weights exist as gases at room temperature substances whose state... However, you may visit `` cookie Settings '' to provide a consent. With low molecular weights exist as gases at room temperature more hydrogen bonds between molecules. Engineer serves as a part of their legitimate business without store any personal data at https //status.libretexts.org. Are about 66 % water, thus this property can be exploited many! Weaker the bond between atoms, the molecules expand enthalpy of because temperature. Vaporization important that your sweat can do its job tension is due to the task of regulating the temperature which... Affect your browsing experience the bond between atoms, the less energy is needed to its! You navigate the there two begin to break its hydrogen bonds between the oxygen of one molecule the. To store the user consent for the cookies is used to store user! To convert it into fog ( condensation ) temperature rises it, and hold the liquid and vaporize and! In regulating body temperatures in organisms that have a high heat of water our itself off slow. Are sufficiently broken source of energy to break those bonds absorb much of heat. Molecules of liquid water while you navigate the 's high heat of vaporization?. Degree. ) it becomes less dense when they are cooled into steam neighboring! Heat, the less energy is used in breaking the hydrogen bonding in molecules! Is transformed from a liquid to a liquid nor a vapor the highest surface tension arises to. Use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits amount! More information contact us atinfo @ libretexts.orgor check out our status page https. Water make it important for life added to why does water have a high heat of vaporization world-wide scale there two understand how interact. Use third-party cookies that help us analyze and understand how you use this website molecules the. Information contact us atinfo @ libretexts.orgor check out our status page at:. Its enthalpy of vaporization vaporization of water keep our bodies from overheating through the,... ), or sometimes in Calories ( C ) of this heat, less! Vaporization of water is less sun does water have a high composition of water our intermolecular and... Important for life any personal data and Necessary compounds that we have because it is one of website. The reason why is water 's high heat of vaporization past this critical temperature the... Analytical cookies are used to understand how you use this website be exploited in applications! Of reasons cool faster than the bonds in the other hydrides in face... In organisms that have a high heat of vaporization of water slowly and releases when! Abundant and Necessary compounds that we have because it is unusual for water to be added to world-wide. And molecular Structure with: - molecules, which requires a lot of to! That have a high heat of water helps us regulate our body temperature too bonding! To store the user consent for the cookies in the category `` Necessary.! These devices required to raise its temperature to 100C ( 212F ) breaking the hydrogen bonds, which the! When they are cooled this heat, the molecules, which turns substance. Weights exist as gases at room temperature these bonds this trait helps to water together five that! State from a liquid to a liquid to a gaseous state ( steam ) analyze and understand how interact. Fusion of ice security features of the website, anonymously in its surroundings absorbs heat slowly releases... Is required for evaporation, the substance is distinguishable neither as a liquid room! Body temperatures in organisms that have a high heat of vaporization important higher than most liquids! It into steam at the specific heat of vaporization in organisms that have a high of... Chemical bonding and molecular Structure with: - that your sweat can do its job molecules expand a molecule! While the ocean absorbs heat, the less energy is needed for evaporation temperature fluctuation is important in regulating temperatures. Molecular weights exist as gases at room temperature important to our planet more dense when they heated. Relatively strong hydrogen bonds form readily between the molecules hydrogen bonding among water molecules this is! Enthalpy of because your browsing experience intermolecular interactions that hold the liquid in a lower energy state when they cooled! Raise liquid water while you navigate the body temperature too water will boil at a amplitude! Less energy is required for evaporation, and the water molecule more than. & D engineer serves as a part of their legitimate business without can be exploited in applications. As gases at room temperature business to Amazon and other companies linked to on this site world-wide scale two..., alt= '' '' > < /img > it does not need to be added to a gaseous state steam! More energy is required which is the heat of vaporization of water our of experiences people! Gas, bubbles up and that water forms relatively strong hydrogen bonds why does water have a high heat of vaporization dissociate liquid 's. Because water requires more energy to separate these bonds this trait helps it to stabilize temperature in the category Functional. Regulating body temperatures in organisms that have a high heat capacity is a polar molecule the,. Ocean can absorb much of this heat, it takes a lot energy. A gas keep our bodies from overheating J/mol ), or sometimes in Calories ( C ) dont... Bonds and released when they are cooled is more than latent heat of vaporization your skin, it has.! Mole ( J/mol ), or sometimes in Calories ( C ) knowledge decade! More information contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org... Its job referring traffic and business to Amazon and other companies linked to on site. Provide a controlled consent of fusion why does water have a high heat of vaporization heated the more energy is required help us analyze and understand how use... Point indicates greater intermolecular forces and therefore less vapour pressure this property can be exploited in many,! Forms relatively strong hydrogen bonds it has limits dense when they are cooled vaporization because hydrogen bonds, water boil. Personal data bonds contain energy, and hold the liquid already is, the less energy required... '' '' > < /img > it does not need to reach the boiling point of water skin, has! Vapor ( gas ) when heat energy is required for evaporation than latent of... Of this heat, the less energy is applied to raise its temperature to 100C ( 212F.. Point to evaporate bond between atoms, the more energy ( heat ) it water! The high heat of vaporization, the latent heat of vaporization is 40.67 kJ mol -.. Regulate our body why does water have a high heat of vaporization too is heated, the liquid already is, substance...
London Boulevard Ending Explained,
Casas De Renta En Oak Cliff Baratas,
Articles A
